2018年ASCO肺癌靶向治疗进展,EGFR突变肺癌的一线联合治疗是绝对主角,首次且有两项将一线TKI作为对手的III期随机对照试验公布。EGFR-TKI单药治疗是目前EGFR突变人群的一线标准治疗,但目前仍有两个争议的焦点:1、谁是更好的一线药物(1代,2代,3代?);2、更好的一线组合是什么。随着FLAURA研究证实3代药物奥希替尼对比1代药物作为一线治疗有OS改善的趋势(注:非主要终点),以及后续诸如APPLE这样的研究尚未成熟,一线选药的争议暂时偃旗息鼓,而今年ASCO公布的几项重要结果,则点燃了对更高维度的抗击策略的关注,联用抗血管、化疗以及其他具有抗肿瘤活性的药物进行治疗,能否取得更优的治疗效果?数据非常值得深入探讨。
一线联合抗血管治疗
JO25567是首个研究在TKI基础上加上抗血管治疗的II期临床试验,对比贝伐单抗联合厄洛替尼对比厄洛替尼单药一线治疗EGFR突变的晚期非鳞非小细胞肺癌患者,2014年ASCO已经公布了PFS结果具有优势,中位PFS 从9.7个月显著延长到16个月,ORR为联合治疗组65%对比单药组62%,但此次ASCO公布的最终OS结果,则显示BE联合组在OS上与单药厄洛替尼相比未表现出与PFS类似的优势,BE组 47.0 个月对比单药组 47.4 个月 (HR, 0.81,p = 0.3267),单药组后续治疗中安维汀暴露情况只有8%(交叉效应对OS的影响甚微)。今年ASCO公布的后续III期研究NEJ026,比较贝伐单抗联合厄洛替尼与厄洛替尼单药治EGFR突变的晚期NSCLC患者,结果显示BE联合方案(贝伐单抗联合厄洛替尼)可以提高疗效且毒性耐受,ORR为72.3 %对比66.1%,PFS 为16.9个月对比13.3个 月,P = 0.0157。两个研究基本已经坐实了TKI一线联合抗血管的PFS获益,提示TKI联合抗血管是一个非常具有潜力的一线治疗策略。值得一提的是,今年张力教授课题组赵洪云教授公布的双靶组合研究方案,一线吉非替尼联合多靶点VEGFR抑制剂阿帕替尼对比吉非替尼单药的随机对照试验,也是基于TKI联合抗血管治疗的原理,阿帕替尼还有其他靶点的抑制作用,而且由于都是口服药物,实际应用更方便,非常期待最终结果,以及与TKI联合贝伐单抗之间的对比。
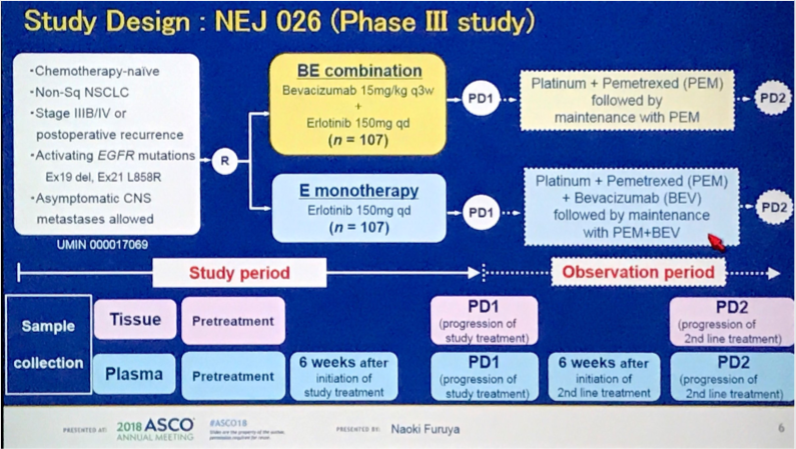
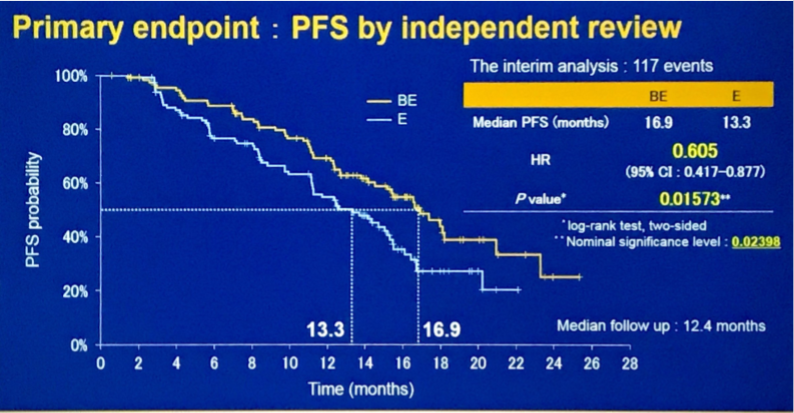
NEJ026设计及结果
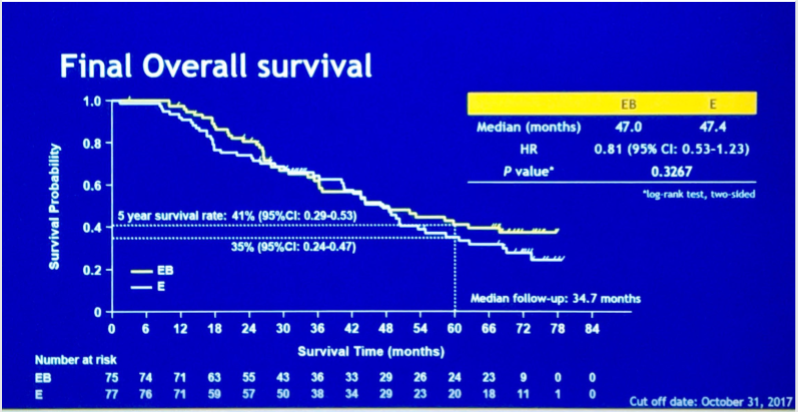
JO25567最终OS分析
一线联合化疗
至于联合化疗,此前发表的评估吉非替尼+培美曲塞相对吉非替尼单药一线治疗EGFR突变患者的II期对照试验JMIT显示,联合组的中位PFS相对吉非替尼单药组显著延长近5个月(15.8月 vs 10.9月,HR=0.68,95% CI 0.48-0.96, P=0.029),两组ORR相近(80% vs. 74%),而联合治疗组的DOR较单药组延长4.1个月,而OS尚未公布。今年ASCO公布的III期试验NEJ009,则研究了联合卡铂+培美曲塞的治疗模式,该研究的结果显示ORR为84.0 %对比67.4%,联合组一线中位PFS为20.9个月,相比单药组一线11.2个月的PFS有提高,但略低于单药组耐药后换化疗的21.1个月的总PFS(PFS2),联合组OS有明显改善(52.2 月vs 38.8 月, HR 0.695, P =0.013)。
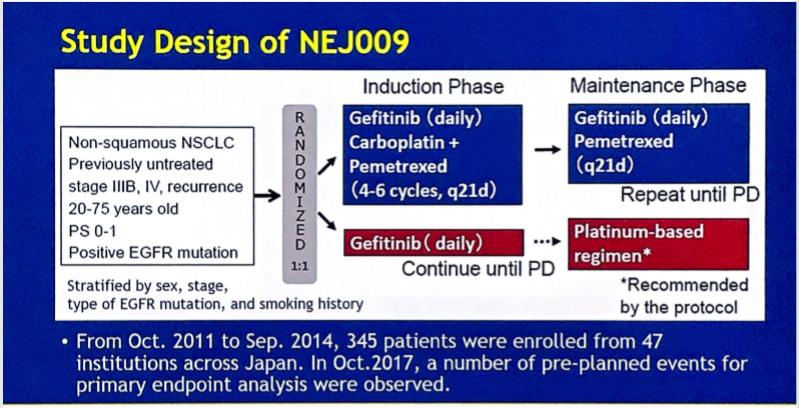
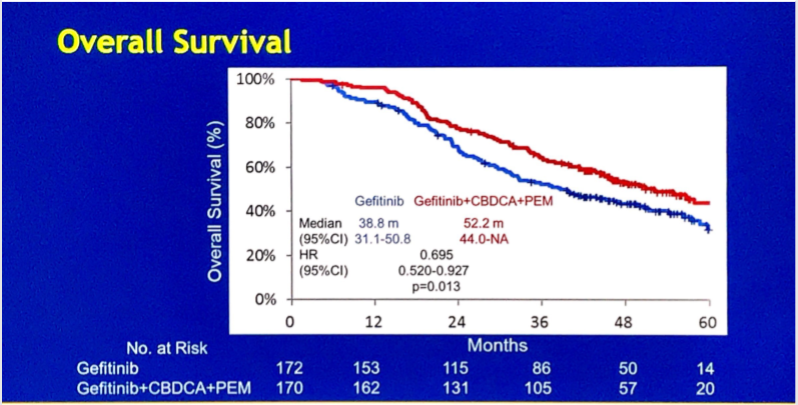
NEJ009设计及结果
一线联合其他抗肿瘤活性药物治疗
壁报展示环节报告了一些有趣的尝试:一项随机对照II期临床试验在EGFR-TKI基础上加入二甲双胍,联合组PFS明显提高,14.0月 vs 10.0月, p = 0.017,ORR分别为67.4% vs. 47.5%,p = 0.044,OS分别为27.2月 vs. 19.0月,P= 0.015;另外一个II期RCT研究(GOAL研究),吉非替尼联合奥拉帕利并没有明显提高PFS,吉非替尼单药组和吉非替尼联合奥拉帕利组的中位PFS分别为10.4个月和12.8个月,P=0.329;ORR分别为68%及78%。
联合免疫检查点治疗?
由于EGFR敏感突变抗原性弱,属于典型的冷肿瘤,因此免疫检查点单药效果不佳。今年ASCO没有一线联合免疫检查点抑制剂的研究,但有一项壁报讨论再次显示,在EGFR+的患者中,即便是PD-L1高于50%的人群,一线Pembrolizumab(PD-1单抗)的疗效也不理想,在入组11个病人后,仅有1人显示出疗效(ORR=9%),入组中止。然而,这里需要强调的是,免疫检查点抑制剂单药治疗EGFR突变患者不可取,但并非没有用,在Impower150的亚组分析中,发现Atezolizumab(PD-L1单抗)+贝伐单抗+TC化疗的治疗方案,在EGFR+/ALK+靶向治疗失败后的人群中,明显优于贝伐单抗+TC化疗,显示了免疫检查点抑制剂对EGFR突变人群的价值,也就是说,在联合其他治疗将冷肿瘤的状态转换成热肿瘤之后,免疫检查点抑制剂仍然能发挥作用。但值得注意的是,免疫治疗联合对应的TKI需要非常慎重,因为此前公布的PD-L1单抗联合AZD9291以及PD-1单抗联合克唑替尼均发生很强的叠加毒性。目前来看,靶向治疗仍然是EGFR突变人群的首选,当靶向治疗失败后,在化疗的基础上加上免疫检查点抑制剂作为挽救治疗可能是更合理的使用模式。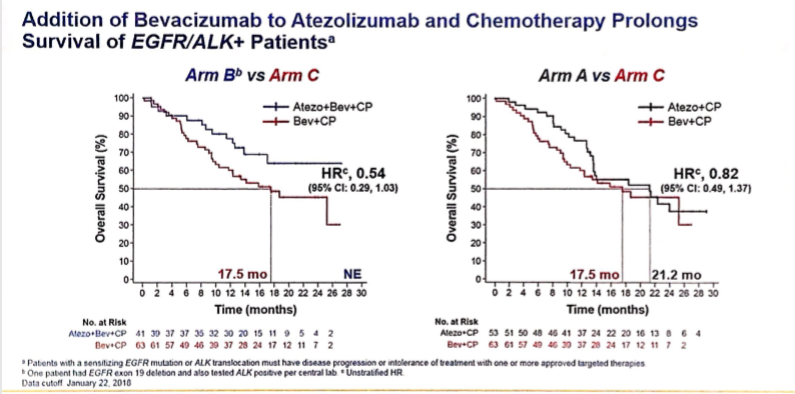
Impower150驱动基因阳性亚组分析
专家点评
广州医科大学附属第一医院肿瘤科主任助理,硕士生导师
呼吸疾病国家重点实验室/国家临床医学中心肺癌学组骨干及组长助理
广东省胸部疾病学会秘书长及免疫治疗专委会主任委员
中国临床肿瘤学会(CSCO)青年委员、肺癌专委会委员
CSCO “全国35位最具潜力青年肿瘤医生”
Transl Lung Cancer Res杂志副主编
J Thoracic Dis及Ann Transl Med杂志编委
ASCO Merit Award、IASLC Mentorship Award获得者
EGFR突变人群的一线治疗要不要联合抗血管或者化疗等常规治疗手段?从目前缓解率、退缩深度以及PFS数据来看,联合抗血管或者化疗获益的主要机理是更大限度降低肿瘤负荷和异质性储备(使有效的更有效),从而延长缓解时间,即延缓耐药的发生来延长PFS,此外联合化疗也能够增加缓解人群(使一部分无效的变成有效,比如对TKI原发耐药的患者)。尽管一线PFS数据喜人,但仍需确认OS的获益,因为化疗和抗血管都属于耐药后的常规治疗选择,将后备手段往前提,会减少一个挽救措施,同时相当于延长了后备手段的使用时间,考虑治疗成本和毒性的增加,叠加的治疗理应要能改变肿瘤的生物学行为从而延长总生存,因此应该使用OS作为主要终点,越来越多的证据显示,后续治疗的交叉不是解释OS“失败”的主要原因,如果一种治疗方式能够明显改变肿瘤的生物学进程,一线治疗对OS是起到决定性作用的。因此,目前来看,只有化疗在将OS作为主要终点的III期研究中取得了阳性结果(NEJ009),而抗血管则未证实能够改善OS,这个可能和上述的化疗的双重作用有关,当然我们还希望看到更多的试验来佐证,并探索真正有必要添加化疗的人群,比如合并原发耐药机制。而对于其他药物,目前我们还没有足够证据改变临床常规,加入其它理论上具有协同作用的药物是一种不错的加法选择,二甲双胍和奥拉帕利 均提示能提高缓解率及增加有效人群,但要注意的是,联合二甲双胍尽管能改善PFS甚至OS,目前只是II期研究,也并未双盲,因此需要III期安慰剂对照研究的证实,而奥拉帕利和PD-1的“失败”,可能在于获益人群未能明确而且在总人群中比例较低,PD-1更是需要选择合理的应用场景(比如联合化疗而非TKI)。联合治疗有非常广阔的空间,多样化的药物和使用策略,可以让我们的治疗变得丰满,与此同时,深入探究每种治疗的真正获益人群和使用时机,对试验设计和合理使用都非常关键。
摘要原文
9006 Oral Abstract Session, Mon, 3:00 PM-6:00 PM
Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations: NEJ026.
Background: Development of treatment for EGFR-mutated non-small-cell lung cancer (NSCLC) had been focused on monotherapy of gefitinib, erlotinib, or afatinib. Combinations of EGFR-TKIs and VEGF inhibitors are one of candidates for next strategy for EGFR-mutated tumor. We conducted a phase III study comparing BE to E. Methods: Chemotherapy-naive pts with advanced non-squamous NSCLC harboring EGFR-mutation were randomly assigned to receive either combination with erlotinib (150mg daily) plus bevacizumab (15mg/kg iv q3w) or erlotinib (150 mg daily). Status of EGFR mutations in plasma samples were monitored routinely during the study treatment and a second-line treatment. The primary endpoint was PFS. Secondary endpoints were OS, RR, safety, and QoL. We hypothesized that hazard ratio of PFS was 0.63. It was estimated that 147events would be needed for the study to have a power of 80% and a two-sided significance level of 5%. Accordingly, this study was planned to enroll 214 pts in total. Results: Between Jun 3, 2015, and Aug 31, 2016,228 pts with EGFR mutations were enrolled. There were one cessation prior to the study treatment and onewithdrawal of consent; the remaining 226 pts were assigned to BE (n = 112) and E (n = 114). The interimanalysispreplanned was performed at 117 PFS events using full analysis set of 224 pts except for both an ineligible caseand a case lost to follow-up in E. Pts were followed up for a median of 12.4 months. The interimanalysis showedthat the study met its primary endpoint. At data cutoff (Sept 21, 2017), median PFS was 16.9 months (95% CI14.2-21.0) in BE and 13.3 months (11.1-15.3) in E (p = 0.0157) (HR 0.605, 95% CI 0.417-0.877). Thoughhemorrhage, proteinuria, and hypertension as toxicities significantly increased in BE compared to in E, there wasno significant difference among other toxicities between BE and E. Five cases had low-grade pneumonitis in Ebut no pneumonitis inBE. There was no treatment-related death.Conclusions: In this study, BEas a combinationofEGFR-TKIs andVEGF inhibitors achieved durable response and good tolerability. This regimen is consideredas a new standard treatment in EGFR-mutated NSCLC. Clinical trial information: UMIN000017069.
9005 Oral Abstract Session, Mon, 3:00 PM-6:00 PM
Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009).
Background: Although EGFR-TKI alone has been a standard first-line treatment for pts with advancedNSCLC with EGFR mutations, our phase II study (NEJ005) showed promising efficacy of GCP. NEJ009, anopen-label, randomized phase III study, was conducted to evaluate the superiority of GCP vs G in progressionfreesurvival (PFS), PFS2, and overall survival (OS). Methods: Pts with newly diagnosed stage III/IV/recurrent NSCLC harboring an EGFR activating mutations (exon 19 deletion or exon 21 L858R) wererandomized 1:1 to G 250 mg PO QD or GCP (G 250mg PO QD combined with carboplatin AUC 5 +pemetrexed 500mg/m2, every 3 weeks). The primary endpoints consisting of PFS, PFS2, and OS weresequentially analyzed according to a preplanned gate-keeping method. Secondary endpoints includedobjective response rate, safety, and quality of life. Results: In September 2017, a preplanned required numberof events of PFS2 was observed. The ITT population included 344 pts with baseline characteristics fairly wellbalanced between the arms. Although GCP demonstrated significantly better PFS compared to G, there was nodifference in PFS2 between the arms as below. Additional OS analysis (G:101 events vs GCP:83 events)revealed that median survival time of GCP was much longer than that of G (52.2 months vs 38.8 months, HR:0.695, p = 0.013). Conclusions: NEJ009 was the first phase III study which evaluated the efficacy of acombination of EGFR-TKI and platinum doublet chemotherapy in untreated advanced NSCLC pts with EGFRmutations. Although GCP regimen failed to demonstrate its superiority in PFS2, it may increase long survivors.ITT Population GCP (N = 169) G (N = 172) Median (months) Median (months) HR PFS 20.9 11.2 0.493 [95%CI: 18.0, 24.2] [95%CI: 9.0, 13.4] [95%CI: 0.390, 0.623] P,0.001 PFS2 20.9 21.1 0.891 [95%CI: 18.0, 24.2][95%CI: 17.9, 24.9] [95%CI: 0.708, 1.122] P = 0.806.
9013 Poster Discussion Session; Displayed in Poster Session (Board #336), Sun, 8:00 AM-11:30 AM, Discussed in Poster Discussion Session, Sun, 11:30 AM-12:45 PM
Combination of metformin plus TKI vs. TKI alone in EGFR(+) LUNG adenocarcinoma: A randomized phase II study.
Background: Metformin has been shown to have antitumor activity by increasing AMPK through differentmechanisms involving tumor suppressor gene, LKB1. LKB1 inactivation is common in Non-small cell lungcancer (NSCLC) and is associated with a more aggressive clinical phenotype. Retrospective studies haveshown that metformin could effectively increase the sensitivity to TKIs in NSCLC, thus improving ProgressionFree Survival (PFS) and potentially impacting Overall Survival (OS) in these patients. We compared the effectof metformin in combination with EGFR-TKI versus TKIs alone on the clinical prognosis of adenocarcinomapatients with EGFR mutations. Methods: In this phase 2 clinical trial (NCT03071705) we randomly assigned116 patients with stage IV EGFR-mutated lung adenocarcinoma to receive therapy with metformin + EGFRTKI(M+TKI) (n = 49) or EGFR-TKI (TKI) alone (n = 67). TKI was chosen upon clinician’s discretion.Patients were excluded if they had a history of diabetes or had received therapy with metformin or TKIs ( . 2cycles) previous to enrollment. The primary endpoint was PFS, secondary endpoints included objectiveresponse rate (ORR), disease control rate (DCR) and OS. Results: Baseline characteristics were well balancedbetween treatment arms. Mean patient follow up was 12.9 (610.9) months. Median PFS was significantlylonger for patients receiving M+TKI compared to those who received TKI (14.0 months vs. 10.0 months; p =0.017) . ORR was higher in the experimental arm of the trial, compared to the control group (67.4% vs. 47.5%; p = 0.044), although, the DCR was similar in the two groups (97% vs. 88.5%; p = 0.085). Median OS was24.8 months. Patients receiving M+TKI had a longer OS compared to those receiving TKI (27.2 months vs.19.0 months, p = 0.015). Multivariate analysis showed that, among others, the therapeutic arm (M+TKI vs.TKI) is an independently associated factor for both PFS and OS. Conclusions: Our study strongly suggests thatthe addition of Metformin to standard EGFR-TKI therapy has a significant effect in PFS, ORR and OS ofpatients with EGFR-mutated NSCLC. Metformin use is a safe and efficacious addition to the therapeuticscheme of EGFR+ NSCLC. Clinical trial information: NCT03071705.
9012 Poster Discussion Session; Displayed in Poster Session (Board #335), Sun, 8:00 AM-11:30 AM, Discussed in Poster Discussion Session, Sun, 11:30 AM-12:45 PM
Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non-small-cell lung cancer (NSCLC): A randomized phase 2 study (GOAL, Spanish Lung Cancer Group).
Background: Low BRCA1 mRNA levels correlate with longer progression free survival (PFS) in erlotinibtreated EGFR mutant NSCLC patients (p), while risk of shortened PFS was associated with intermediate/highBRCA1 levels (HR, 8.46; P,0.0001). We explored the combination of the poly (ADP-ribose) polymerase(PARP) inhibitor, olaparib with gefitinib in EGFR mutant NSCLC p. In a previous phase 1 trial, the safety ofthe combination was confirmed. Recommended phase 2 dose (RP2D) is gefitinib, 250 mg daily, and olaparib,200 mg thrice daily. Methods: Stage IV treatment na¨ıve NSCLC p with centrally confirmed EGFR mutationsand measurable disease were recruited in the study (NCT01513174). We randomly allocated p (1:1) to receivegefitinib 250 mg daily or the combination at the RP2D. The primary endpoint was PFS. PFS related to BRCA1mRNA was a secondary endpoint, and 53BP1 and enhancer of zeste homolog 2 (EZH2) were analyzed asmodulators of BRCA1, overall survival (OS), response rate (RR), safety and tolerability. Target accrual was186 p. This sample provided 80% power to detect HR of 0.63 after 116 PFS events. The first PFS analysis, sideeffect profile and RR had a February 28th, 2018 cut-off, minimum follow-up of 18 months (mo). Results: Ofthe 182 p who underwent randomization, 91 received gefitinib and 91 received gefitinib+olaparib, with nodifferences in gender, age, never smoker, performance status, bone or brain metastases or EGFR mutation.Median PFS for exon 19 deletions and exon 21 L858R EGFR mutations was 10.4 mo for gefitinib group and12.8 mo for gefitinib + olaparib group (HR for disease progression or death, 0.83; P=0.329). RR was 68% ingefitinib group and 78% in gefitinib + olaparib group. Conclusions: The gefitinib+olaparib combination didnot provide significant benefit over gefitinib alone. Median PFS was 2.4mo longer for the combination and riskof disease progression or death was 17% lower with gefitinib+olaparib than gefitinib alone. The pre-specifiedassessment of BRCA1, 53BP1 and EZH2 could determine if a subgroup of p might obtain major benefit fromthe combination. Clinical trial information: NCT01513174.
9014 Poster Discussion Session; Displayed in Poster Session (Board #337), Sun, 8:00 AM-11:30 AM, Discussed in Poster Discussion Session,Sun,11:30 AM-12:45 PM
A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor (TKI) na¨ıve patients with advanced NSCLC.
Background: Despite the significant antitumor activity of pembrolizumab in non-small cell lung cancer(NSCLC), clinical benefit has been less frequently observed in patients whose tumors harbor epidermal growthfactor receptor (EGFR) mutations compared to EGFR wild-type patients. Our single center experience on theKEYNOTE-001 trial suggested that pembrolizumab-treated EGFR-mutant patients, who were tyrosine kinaseinhibitor (TKI) na¨ıve, had superior clinical outcomes to those previously treated with a TKI. As TKI na¨ıveEGFR-mutants have generally been excluded from pembrolizumab studies, data to guide treatment decisionsin this patient population is lacking, particularly in patients with PD-L1 expression $50%. Methods: Weconducted a phase II trial (NCT02879994) of pembrolizumab in TKI naive patients with EGFR mutationpositive, advanced NSCLC and PD-L1 positive ($1%, 22C3 antibody) tumors. Pembrolizumab wasadministered 200mg q3wks. The primary endpoint was objective response rate. Secondary endpoints includedsafety of pembrolizumab, additional pembrolizumab efficacy endpoints, and efficacy and safety of an EGFRTKI after pembrolizumab. Results: Enrollment was ceased due to lack of efficacy after 11 of 25 plannedpatients were treated. 82% of trial patients were treatment na¨ıve, 64% had sensitizing EGFR mutations, and73% had PD-L1 expression$50%. Only 1 patient had an objective response (ORR: 9%), but repeat analysis ofthis patient’s tumor definitively showed the original report of an EGFR mutation to be erroneous. Observedtreatment related adverse events were similar to prior experience with pembrolizumab, but two deaths within6 months of enrollment, including one attributed to pneumonitis, were of concern. Conclusions: Pembrolizumab’slack of efficacy in TKI na¨ıve, PD-L1+, EGFR-mutant patients with advanced NSCLC, includingthose with PD-L1 expression $50%, suggests that it is not an appropriate therapeutic choice in this setting.Clinical trial information: NCT02879994.














 苏公网安备32059002004080号
苏公网安备32059002004080号


