点评:王玉栋

河北医科大学第四医院肿瘤内科 主治医师
一项II期随机临床试验:扰频器治疗化疗所致神经病变(ABS:10016)
First Author: Charles L. Loprinzi, Mayo Clinic, Department of Oncology
研究背景
既往的研究数据表明扰频器可能有益于化疗所致周围神经病变(CIPN)的患者。
试验方法
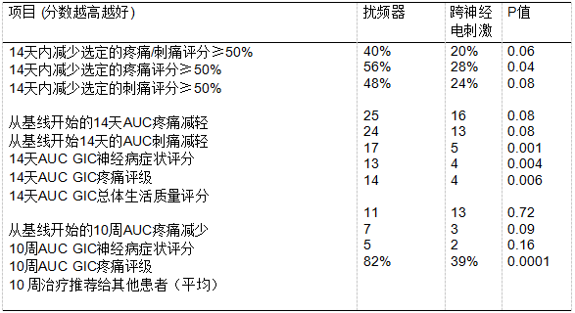
入组条件为既往接受过可以导致神经毒性的化疗患者,且CIPN至少存在3个月。在入组前一周内,受试者CIPN相关的刺痛或疼痛至少达到4/10。将受试者随机分配到两组,分别接受两周的扰频器治疗或TENS(经皮神经电刺激)治疗。利用EORTC CIPN 20量表、受试者总体变化印象量表(GIC)以及数字模拟量表对患者的治疗效果进行评估。研究人员会在每个治疗日之后以及治疗后连续8周每周询问患者,他们是否会向其他人推荐自己所接受的治疗。研究的主要终点是治疗两周后与基线值相比疼痛或刺痛评分降低50%以上患者的百分比(针对每名患者在基线时选择的认为对自己影响最大的项目)。统计方法为使用卡方检验估计和比较症状评分降低50%的患者比例,使用t检验比较曲线下面积(AUC)。
结果
研究共入组50名患者,随机分两组,每组25名,但这不是一项III期临床研究,入组病例较少,有一定局限性。
结论
研究结果支持扰频器可能会将CIPN症状降低到中等程度,也提示了扰频器治疗CIPN值得进一步探索。 临床试验信息:NCT01290224。(研究数据见下表)
一项随机双盲III期临床研究:单唾液酸四己糖基神经节苷脂(GM1)与安慰剂对比治疗胃肠道肿瘤患者奥沙利铂相关周围神经毒性(TJMUCH-GI-001)(ABS:10017)
第一作者:周礼鲲,天津医科大学肿瘤研究所,国家癌症临床研究中心天津市肿瘤临床研究中心,天津医科大学肿瘤防治重点实验室
背景
神经毒性是奥沙利铂最常见的剂量限制性毒性,目前尚未有剂量累积致感觉神经病变的治疗方法。 本试验旨在研究单唾液酸四己糖基神经节苷脂(GM1)治疗胃肠癌患者奥沙利铂相关周围神经毒性(OIPN)的功效。
方法
此为单中心研究,将奥沙利铂相关周围神经毒性的胃肠道肿瘤患者(TJMUCH)以随机双盲的方法以1:1的比例随机接受GM1或安慰剂治疗。 入组条件为患者在接受奥沙利铂为基础的化疗期间或之后持续存在OIPN。 入组后继续应用奥沙利铂的患者每周期接受安慰剂或GM1×7天治疗,已停用奥沙利铂的患者每3周接受安慰剂或GM1×14天治疗。GM1剂量为60mg/日 每3周或40mg 每2周。 研究持续直到目测类比评分(VAS)降低30%或在奥沙利铂应用结束后再进行两次治疗评分保持不变。研究主要终点是修正的EORTC QLQ-CIPN20(MCIPN20)评分降低≥30%,次要终点是VAS改善≥30%、CTCAE改善≥1级。接受1周期以上治疗的患者接受分析。 应用卡方检验进行统计分析。
结果
2015年5月至2017年12月,GM1组入组73例患者,安慰剂组入组72例。 GM1组39例(53%)患者及安慰剂组10例(14%)患者MCIPN20降低≥30%(RR = 3.85,95%CI,2.08-7.11,P <0.0001)。 GM1组36例(49%)患者及安慰剂组16例(22%)患者VAS改善≥30%(RR = 2.22,95%CI,1.36-3.623,P = 0.001)。GM1组的中位治疗时间为2周期(1-7)。 在试验中两组患者中大多数患者(GM1组为89%,安慰剂组为83%)继续接受奥沙利铂治疗。 两组之间的CTCAE和急性神经毒性分级并没有显著差异。 没有发生与GM1相关的≥G3的不良事件。
结论
GM1可有效降低胃肠肿瘤患者的奥沙利铂相关外周神经毒性。研究发现患者的临床获益与停用奥沙利铂无关。 试验:NCT02486198
一项全国纵向研究:接受紫杉醇治疗前根据乳腺癌患者身体锻炼情况预测短期和长期化疗相关周围神经病变(CIPN)的严重程度(ABS:10018)
第一作者:Ian Kleckner,罗切斯特大学医学中心
研究背景
CIPN是一种剂量限制性毒性,目前并没有明确的治疗方法,并且我们对致CIPN的危险因素所知有限。 在化疗过程中进行体力活动可能会改善CIPN,但尚不明确治疗前进行体力活动是否有助于防止或改善CIPN。本研究针对接受紫杉醇治疗前的乳腺癌患者的身体锻炼情况,分析是否影响短期和长期CIPN症状的严重程度。
研究入组了200例非转移性乳腺癌患者(52±10岁)。有接受紫杉醇治疗意向的患者接受三次CIPN (将过去一周的麻木/刺痛严重程度分为0-10)评估,分别为在应用紫杉醇前1周内、应用紫杉醇后1个月和6个月进行评估。研究中将紫杉醇前期神经病变、年龄、BMI、糖尿病(是/否)和紫杉醇剂累积量等因素进行控制后,我们使用线性回归来检验患者应用紫杉醇前的体力活动情况(Aerobic中心纵向研究)来评估体力活动是否可预测的CIPN症状(应用紫杉醇后1月或6月内)的严重程度。
CIPN症状严重程度在紫杉醇应用前后(+3.6单位; p<0.001)和应用前和之后6个月随访(+2.08单位; p<0.0001)显著改善。 考虑到0.5个单位的变化即具有临床意义,这提示CIPN的严重程度升高明显。应用紫杉醇前每天额外15分钟的体力活动可显著改善应用紫杉醇后(-0.5; p = 0.0002)以及6个月随访(-0.25; p = 0.09)过程中CIPN的症状。研究过程中在控制了紫杉醇前期神经病变、体力活动、BMI、糖尿病和紫杉醇剂量等因素后,患者年龄每增加10岁就会显著增加应用紫杉醇后(+0.8,p<0.0001)以及6个月随访期(+0.9; p<0.0001)CIPN症状严重程度。
结论
应用紫杉醇前进行更多体力活动的乳腺癌患者在紫杉醇治疗后及之后6个月的随访中CIPN症状更轻微。 由于CIPN的严重程度随年龄增加而增加,因此体力活动对老年患者可能尤其重要。临床医生在处方紫杉醇时应建议患者治疗前进行体力活动,这可能会提高患者治疗耐受性。
专家点评

中国医疗保健国际交流促进会肿瘤姑息治疗与人文关怀分会常委
中国康复技术转化及发展促进会精准医学与肿瘤康复专业委员会委员
中国医疗保健国际交流促进会神经内分泌肿瘤分会(CNETS)青年委员会委员
中国医药教育协会肺部肿瘤专业委员会青年委员
北京医学奖励基金会肺癌医学青年专家委员会常委
河北省抗癌协会肿瘤标志专业委员会常委
河北省抗癌协会肿瘤内科专业委员会委员兼秘书、肺癌多学科协作组秘书
M.D. Anderson Cancer Center访问学者
以上3项研究中,其中一项药物性治疗研究来自 天津医科大学肿瘤医院的周礼鲲教授,采用神经损伤后继发性神经退化保护剂-单唾液酸四己糖神经节苷脂治疗CIPN ,有效降低胃肠肿瘤患者的奥沙利铂相关外周神经毒性;一项非药物性干预扰频器治疗CIPN,可能会将CIPN症状降低到中等程度;另一项研究显示在应用紫杉醇之前体力活动可显著减轻应用紫杉醇后CIPN的症状。
CIPN是化疗药物常见的一种剂量限制性毒性,约60%肿瘤患者会发生CIPN,且20-30%的患者会长期持续存在,严重影响患者的生活质量。特别是奥沙利铂和紫杉醇等药物治疗的患者。尽管如此,CIPN在临床工作中往往被忽视,只有少数患者在其医疗记录中得到了明确的CIPN诊断。而且,由于缺乏证据级别高的大型随机对照研究的数据,至今FDA尚未批准任何明确的预防和治疗方法或药物。目前,CIPN相关研究主要分为药物性干预和非药物性干预两大类。度洛西汀是ASCO唯一适度推荐的治疗CIPN的药物,但仍未推荐预防CIPN的药物。而α-2-δ受体拮抗剂,如加巴喷丁,以及阿片类药物治疗CIPN均未获得大型试验的验证。对于治疗CIPN的大部分药物旨在缓解症状,包括疼痛和感觉异常,但并不十分有效。而用于CIPN患者的非药物干预主要包括运动,神经电刺激,针灸,按摩和足浴等,也仅仅是改善了CIPN症状和体征,缓解了CIPN疼痛程度,但并未显著逆转CIPN转归和预防CIPN的发生。
随着癌症幸存者生存期的逐步延长,需要更加重视CIPN等肿瘤治疗所导致的长期副作用的潜在风险和负担。一方面应深入全面研究CIPN的病因学和发病机制,以便开发有效的,基于机制的CIPN改善干预措施,并且这些干预措施不应该干扰化疗的抗肿瘤作用。另一方面,应尽快制定和完善适当和广泛使用的CIPN评估工具和评价标准,便于临床医生和患者进行前瞻性监测、评估和防治。
附摘要原文:
ABS:10016
Poster Discussion Session; Displayed in Poster Session (Board #4), Mon, 1:15 PM-4:45 PM, Discussed in Poster Discussion Session,
Mon, 4:45 PM-6:00 PM
Scrambler therapy for established chemotherapy-induced neuropathy: A randomized phase II trial. First Author: Charles L. Loprinzi, Mayo Clinic, Department of Oncology, Rochester, MN
Background: Pilot data support that Scrambler therapy may benefit patients with Chemotherapy-induced peripheral neuropathy (CIPN).
Methods: Patients with CIPN for at least 3 months were eligible if the patient had finished previous neurotoxic chemotherapy. They needed to have CIPN-related tingling or pain of at least 4/10 during the week prior to registration. Patients were randomized to receive Scrambler therapy versus TENS (trans-electrical nerve stimulation), for 2 weeks. Patient- reported outcomes were utilized, including the EORTC CIPN 20 instrument, the Subject Global Impression of Change (GIC) scale, and a number of 0-10 numerical analog scales regarding appli- cable symptoms. After each treatment day, and weekly for 8 more weeks, patients were asked whether they would recommend their therapy to others. The primary chosen endpoint was the percentage of patients who achieved more than 50% reduction in pain or tingling scores (based on which item was selected most problematic at baseline for each patient), from baseline values, after 2 weeks of therapy. The proportion of patients with $50% reduction in symptom scores were estimated and compared using the chi-square test. The areas under the curves (AUC) were compared using the t-test.
Results: 50 patients were randomized, 25 per arm. Data are provided in the Table. P values are provided, understanding that this is not a well-powered phase III trial.
Conclusions: These results support that Scrambler therapy decreases CIPN symptoms, to a moderate degree. Further exploration of this approach is in- dicated. Clinical trial information: NCT01290224.
ABS:10017
Poster Discussion Session; Displayed in Poster Session (Board #5), Mon, 1:15 PM-4:45 PM, Discussed in Poster Discussion Session,
Mon, 4:45 PM-6:00 PM
Randomized, double-blind, phase III trial of monosialotetrahexosylganglioside versus placebo in GI cancer patients with oxaliplatin induced peripheral neurotoxicity (TJMUCH-GI-001). First Author: Zhou Likun, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer Tianjin’s Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy Tianjin Medical University, Tianjin, China
Background: Neurotoxicity is the most common dose-limiting toxicity of oxaliplatin. There is no treatment for cumulative sensory neuropathy. This trial is designed to study the efficacy of Monosialotetrahexosylganglioside (GM1) in GI cancer patients with oxaliplatin-induced peripheral neurotoxicity (OIPN).
Methods: In this single center (TJMUCH),double-blind, phase Ⅲ trial, patients were randomized in a 1:1 ratio to receive GM1 or placebo. Patients with OIPN persisting during or after oxaliplatin-based chemotherapy were eligible. The patients who remained on oxaliplatin after enrollment, received concurrent placebo or GM1 x 7 days with each chemotherapy cycle. The patients who stopped taking oxaliplatin, were treated with placebo or GM1 x 14 days every 3 weeks. GM1 was dosed at 60mg daily for every 3-week or 40mg daily for every 2-week schedule. Trial was continued until visual analogy score (VAS) decreased by ≥30% or stayed unchanged after two more treatments beyond completion of oxaliplatin. The primary endpoint was reduction of modified EORTC QLQ-CIPN20 (MCIPN20) score by ≥30%. Secondary endpoints were improvement of VAS by ≥30%, CTCAE grade by≥1. Patients who received 1 treatment cycle were included in the analysis. Chi-square tests were used for statistical analysis.
Results: From May 2015 to Dec 2017, 73 patients were enrolled in GM1 and 72 in placebo arm. 39 (53%) patients in GM1 and 10 (14%) in placebo arm achieve ≥30% reduction in MCIPN20 (RR = 3.85, 95% CI, 2.08-7.11, P<0.0001). 36 (49%) patients in GM1 and 16 (22%) in placebo arm had ≥30% improvement of VAS (RR = 2.22, 95% CI,1.36-3.623, P = 0.001). The median treatment cycles of GM1 was 2 (range,1-7). Majority of patients in both arms (89% in GM1 and 83% in placebo) continued receiving oxaliplatin on the trial. There were no significant differences in CTCAE and acute neurotoxicity grading between the two arms. There was no ≥G3 GM1 related adverse events.
Conclusion: GM1 effectively reduces OIPN in GI cancer patients. The observed clinical benefit is independent of oxaliplatin discontinuation. Trial: NCT02486198 (Drug was afforded by Qilu Pharmaceutical Co., Ltd, China). Clinical trial information: NCT02486198.
ABS:10018
Poster Discussion Session; Displayed in Poster Session (Board #6), Mon, 1:15 PM-4:45 PM, Discussed in Poster Discussion Session,
Mon, 4:45 PM-6:00 PM
Pretreatment physical activity to predict short- and long-term chemotherapy-induced peripheral neuropathy (CIPN) in a nationwide longitudinal study of paclitaxel for breast cancer. First Author: Ian Kleckner, University of Rochester Medical Center, Rochester, NY
Background: CIPN is a dose-limiting toxicity with no established treatments and limited knowledge of risk factors. Exercise during chemotherapy may mitigate CIPN, but it is unknown whether pre-treatment physical activity protects against CIPN. This secondary analysis examines whether physical activity before paclitaxel predicts short- and long-term CIPN symptom severity.
Methods: 200 women with non-metastatic breast cancer (52±10 years) receiving paclitaxel with curative intent rated their CIPN (0-10 severity of numbness/tingling in the past week) three times—within 1 week pre- paclitaxel, and within 1 month and 6 months post-paclitaxel. We used linear regression to test whether pre-paclitaxel patient-reported physical activity (Aerobic Center Longitudinal Study) predicted CIPN symptoms (either within 1 month or 6-months post-paclitaxel) controlling for pre-paclitaxel neuropathy, age, BMI, diabetes (yes/no), and cumulative paclitaxel dose.
Results: CIPN symptom severity increased significantly from pre- to post-paclitaxel (+3.6 units; p< 0.001) and from pre- to 6-month follow-up (+2.08; p<0.0001). This is a high level of development of CIPN considering that a 0.5-unit change is clinically significant. Each additional 15 min/day of physical activity pre- paclitaxel was associated significantly less severe CIPN symptoms at post- paclitaxel (-0.5; p= 0.0002) and 6 months follow-up (-0.25; p= 0.09). Each additional 10 years of age was associated with significantly more severe CIPN symptoms at post-paclitaxel (+0.8, p<0.0001) and 6-month follow-up (+0.9; p<0.0001) controlling for pre-paclitaxel neuropathy, physical activity, BMI, diabetes, and paclitaxel dose.
Conclusions: Breast cancer patients who are more physically active pre-paclitaxel experience less severe CIPN immediately and 6 months post-paclitaxel. Physical activity may be especially important for older patients because CIPN severity increases with age. Clinicians prescribing paclitaxel should ask patients about pre-treatment physical activity levels because it may lead to greater treatment tolerability.















 苏公网安备32059002004080号
苏公网安备32059002004080号


