治疗性单克隆抗体介导Fcγ受体依赖性机制,包含ADCC和ADCP等固有免疫和适应性免疫机制。FcγRs多态性会影响Fc结合亲和力,Fcγ受体依赖性机制与治疗结果密切相关。Fc-糖工程或Fc-蛋白质工程可改善Fc结合亲和力,增强Fcγ受体依赖性机制。Fc蛋白工程-马吉妥昔单抗已被证实有效且安全,2022年CSCO BC指南已将马吉妥昔单抗联合化疗作为晚期HER2+乳腺癌三线治疗推荐方案。本期我们特邀请安徽医科大学第一附属医院杜瀛瀛主任和戴映医生就《Fcγ受体在乳腺癌HER2靶向治疗中的作用》进行解读。

前言
曲妥珠单抗是首个获批用于癌症治疗的人源化单克隆抗体,也是首个获批用于治疗乳腺癌的生物制剂。其临床活性包括非免疫机制和免疫介导机制。抗原结合域(Fab)与肿瘤细胞表面的HER2受体结合,干扰HER2信号传导,发挥抑制肿瘤增殖的作用1-2。结晶区域(Fc)与免疫细胞表达的Fc受体(FcRs)结合,FcRs介导先天性和适应性免疫的交叉应答,并表现出不同活化特性的多态性变体(见图1)3-8。
 图1,抗HER2 mAb的作用机制:抗增殖作用和免疫激活。
图1,抗HER2 mAb的作用机制:抗增殖作用和免疫激活。
Fcγ 受体
Fcγ受体是FcRs的主类,由多种亚型组成(见图2)。低亲和力FcγRs包括两种活化受体FcγRIIIa(CD16A)、FcγRIIa(CD32A)和独有的抑制型受体FcγRIIb(CD32B)9。低亲和力FcγRs结合力在30nM-1000nM之间,作为体内抗体功能的重要介质,包括抗体依赖性细胞毒性(ADCC)、抗体依赖性细胞吞噬(ADCP)以及细胞因子和趋化因子的诱导10。

图2,FcγRs在功能、细胞分布、免疫应答、信号修饰和IgG分子亲和力的差异
Fcγ受体多态性
多数的Fcγ受体的多态性与等位基因变异已被证实11-12。FcγRIIa和FcγRIIIa等位基因多态性产生的变体与IgG Fc结合亲和力不同,Fc结合亲和力和ADCC相对效应密切相关。FcγRIIa 131H(组氨酸)和FcγRIIIa 158V(缬氨酸)对IgG Fc的亲和力高于FcγRIIa 131R(精氨酸)和FcγRIIIa 158F(苯丙氨酸)。Fcγ受体多态性普及率详见表1,其中FcγRIIIa-158V纯合子人群占比12%,FcγRIIa-131H纯合子在白种人和黑人占比约27%,亚洲人约占60%13。
表1,FcγRIIIa、FcγRIIa和FcγRIIb多态变体的普及率(基于现有文献)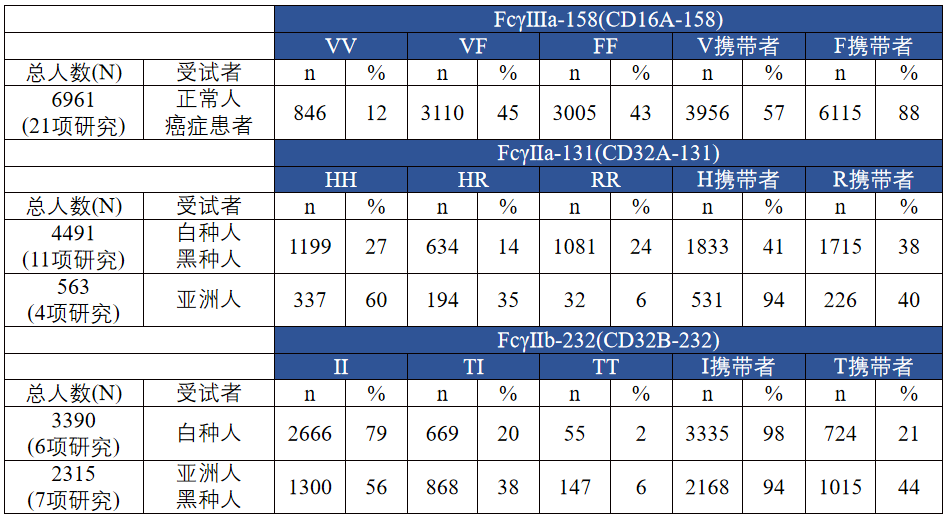
治疗性mAbs介导的ADCC
抗体依赖性细胞毒作用(ADCC)是一种由固有免疫细胞介导的Fc依赖性机制10 14。NK细胞表达的的FcγRIIIa受体与抗体Fc区域结合形成免疫突触,触发穿孔素和颗粒酶的分泌,从而诱导肿瘤细胞死亡(见图3)15-16。体外实验表明,利妥昔单抗、奥法木单抗、维妥珠单抗、奥瑞珠单抗、曲妥珠单抗和 西妥昔单抗等治疗性单克隆抗体都具有人外周血单核细胞(PBMC)或NK细胞介导ADCC活性 3 17-20。体外试验证明ADC药物介导的ADCC效应相当于曲妥珠单抗,因此抗体偶联药物T-DM1、DS-8201、SYD985的Fc段介导ADCC活性较其源抗体未有改变21-23。
帕妥珠单抗抗原结合位点不同于曲妥珠单抗,其抗原结合表位与二聚化结构域重叠,可有效抑制HER2同源和异源二聚化24-25。帕妥珠单抗携带与曲妥珠单抗相同的野生型Fc结构域;曲妥珠单抗联合帕妥珠单抗会增加HER2+肿瘤细胞上FcγRs结合位点密度,增强NK细胞介导的ADCC和巨噬细胞介导的ADCP抗肿瘤效应16。 已知KPL-4细胞可抵抗曲妥珠单抗和帕妥珠单抗的直接抗增殖效应;在HER2过表达人KPL-4乳腺癌异种移植瘤裸鼠模型中,曲妥珠单抗联合帕妥珠单抗具备有效的抗肿瘤作用,其机制完全归于Fc依赖性作用25。CLEOPATRA和NeoSphere临床研究进一步证实曲妥珠单抗联合帕妥珠单抗显著的抗肿瘤活性26-27。
紫杉烷是破坏细胞分裂的微管抑制剂,通过诱导免疫原性细胞死亡和触发免疫刺激应激反应对免疫系统产生影响28。体外研究发现在HER2+乳腺癌患者中,曲妥珠单抗联合紫杉烷治疗可增加循环NK细胞上的NKG2D表达,并增强曲妥珠单抗介导的ADCC29。NeoSphere研究进一步证实曲妥珠单抗或曲妥珠单抗+帕妥珠单抗联合紫杉烷在该类人群中的抗肿瘤活性2 27。
Fc区糖基化也可影响受体结合和ADCC效应。研究发现Fc区岩藻糖基化聚糖高,会使Fc区与FcγRIIIa亲和力减弱,ADCC效应降低。Fc区甘露糖聚糖高,会使Fc区与FcγRIIIa的亲和力增加,ADCC效应增强30。曲妥珠单抗生物类似物SB3 vs曲妥珠单抗新辅助治疗HER2+早期乳腺癌患者的III期试验示SB3组的EFS更佳。其原因在于研究药物曲妥珠单抗Fc区高岩藻糖基化聚糖31。

图3,经典的颗粒酶/穿孔素介导的细胞凋亡途径
增强治疗性mAbs的ADCC活性的策略
Fc糖工程可特异性的提高FcγRIIIa结合亲和力,增强ADCC32-33。体外研究证实,在HER2扩增乳腺癌FcγRIIIa-158F转基因免疫缺陷小鼠中,无岩藻糖基化曲妥珠单抗可增强NK细胞介导的ADCC效应,延缓肿瘤进展和改善实体瘤患者抗肿瘤反应34-35。
Fc蛋白工程也可提高FcγRIIIa结合亲和力,增强ADCC36。马吉妥昔单抗是Fc工程改造的抗HER2单克隆抗体,改变Fc结构域中5个氨基酸(L235V/F243L/R292P/Y300L/P396L),从而增加激活型受体FcγRIIIa亲和力,降低抑制型受体FcγRIIb亲和力。体外研究显示,马吉妥昔单较传统的曲妥珠单抗抗ADCC明显增强。在HER2扩增乳腺癌FcγRIIIa-158F转基因免疫缺陷小鼠中,马吉妥昔单抗具备更强的抗肿瘤活性37-38。
选择性去除唾液酸的抗体-酶结合物也可改善ADCC;曲妥珠单抗唾液酸酶结合物以HER2依赖性方式去唾液酸化肿瘤细胞,增强ADCC39。
增强ADCC的方法还包括联合治疗,例如联合拉帕替尼和双特异性抗体40-42。
治疗性mAbs介导的ADCP
抗体依赖性细胞吞噬作用(ADCP)是由巨噬细胞、单核细胞或中性粒细胞等吞噬细胞介导的另一种重要的Fc依赖性免疫机制。原发性乳腺癌肿瘤相关巨噬细胞(TAM)加快肿瘤进展,TAM浸润增加与不良预后相关43。TAMs表达高水平的激活型受体FcγRIIa和FcγRIIIa,保留抗体依赖性吞噬肿瘤细胞的能力44。体外研究显示,FcγRIIIa对ADCP活性的影响大于FcγRIIa45。小鼠乳腺癌异种移植研究中,曲妥珠单抗ADCP活性取决于肿瘤组织中巨噬细胞的募集46。
增强ADCP的策略
Fc糖工程和Fc蛋白工程,增强激活型受体FcγRIIIa结合亲和力,相较曲妥珠单抗,改良版Fc介导的ADCP效应更强47-48。CD47通过结合并触发巨噬细胞SIRPα的信号来抑制吞噬作用,发出“不要吃我”信号49。曲妥珠单抗联合抗CD47(MIAP410),可增强ADCP效应,改善抗肿瘤效应并延长生存期50。另一项研究发现,曲妥珠单抗联合抗CD47(Hu5F9-G4)也可增强ADCP,在治疗中断后仍存在肿瘤抑制作用51。另外曲妥珠单抗联合B7-H4阻断剂或组蛋白脱乙酰酶抑制剂也可增强ADCP52-53。
FcγR多态性与曲妥珠单抗治疗HER2+乳腺癌的研究结果相关
CHER-LOB研究,对121例可手术HER2+乳腺癌患者进行曲妥珠单抗+化疗组(A组),拉帕替尼+化疗组(B组)和曲妥珠单抗+拉帕替尼+化疗(C组)新辅助治疗,主要终点是病理完全缓解率(pCR)。结果显示,C组pCR率高且差异有统计学意义。FcγRIIIa-158V携带者,C组pCR率高于A组(67%vs27%)和B组(67%vs22%)且差异有统计学意义(p<0.05)但对于FcγRIIIa158F纯合子,C组pCR率与A组(42%vs25%)和B组(42%vs50%)的差异无统计学意义(p>0.05)54。一项纳入15例早期HER2+乳腺癌患者,曲妥珠单抗+化疗的新辅助研究结果发现,FcγRIIa-131H纯合子亚组的pCR率高于FcγRIIa-131R携带者(71%vs0%)55。但另一项纳入26例HER2+乳腺导管癌患者,曲妥珠单抗+化疗的新辅助研究结果发现,FcγRIIa-131R纯合子pCR率高于FcγRIIa-131H携带者(50%vs25%)。FcγRIIa-131R纯合子与pCR率显著相关(p=0.012),但FcγRⅢa-158多态性与pCR率无关(p=0.590)56。
NSABP B-31研究显示曲妥珠单抗+化疗辅助治疗HER2阳性乳腺癌可改善预后57,对本研究1156例患者回顾性分析发现,FcγRIIIa-158F纯合子获益不大(HR=0.71,95%CI 0.51-1.01),但FcγRIIIa-158V携带者DFS获益明显(HR=0.31,95%CI 0.22-0.43)58。值得注意的是,BCIRG-006研究和NCCTG-N9831研究大型回顾性分析(>1000人)并未发现FcγRIIIa或FcγRIIa多态性与预后的相关性59-60。BCIRG-006研究FcγR多态性分析发现联合曲妥珠单抗未能改善预后59。NCCTG-N9831研究中FcγRIIb-232I纯合子,曲妥珠单抗+化疗组DFS优于单独化疗(p<0.0001)60。但两项研究都存在抽样偏倚,可能会降低FcγR多态性与治疗结果之间的相关性。UNICANCER-PACS04研究发现,曲妥珠单抗+化疗组治疗FcγRIIa-131H携带者5年无事件生存率高于FcγRIIa-131H纯合子(90%vs70%,p=0.028),与FcγRIIIa-158多态性无关61。
曲妥珠单抗+紫杉烷治疗HER2+转移性乳腺癌(N=54)的回顾性分析结果显示,相较FcγRIIIa-158F携带者,FcγRIIIa-158V纯合子ORR、PFS以及体外ADCC活性更高62。SOPHIA研究FcγRIIIa-158等位基因亚组分析显示,FcγRIIIa-158F携带者中马吉妥昔单抗+化疗组PFS优于曲妥珠单抗+化疗(HR=0.68,95%CI 0.52-0.90),但FcγRIIIa-158V纯合子中未观察到差异(HR=1.78,95%CI 0.87-3.62)。FcγRIIa-131基因型和FcγRIIb-232基因型都未观察到相关性63。另一项曲妥珠单抗治疗转移性HER2+乳腺癌小型研究(N=35)发现,相较FcγRIIa-131R携带者,FcγRIIa-131H纯合子ORR更高(40%vs10%)、PFS更长(9.2月vs3.5月)64,曲妥珠单抗+化疗治疗转移性胃癌回顾性分析也发现与FcγRIIa-131R携带者相比,FcγRIIa-131H纯合子的PFS更高(HR=0.36,95%CI 0.16-0.82)65。
FcγRs促进适应性免疫
众所周知,先天性免疫激活有助于T细胞介导的适应性抗肿瘤反应14。激活的NK细胞、巨噬细胞及其他免疫细胞,会引起肿瘤细胞溶解,释放肿瘤抗原,摄取并被抗原提呈细胞识别,启动适应性T细胞介导的抗肿瘤作用66-67。活化的NK细胞因子促进巨噬细胞和树突状细胞活化,进而刺激细胞毒性T细胞向肿瘤内空间迁移。FcγRs也可在适应性免疫反应中发挥作用16 67。 mAbs治疗诱导ADCC和ADCP产生肿瘤抗原免疫复合物,FcγR依赖性机制有助于抗原呈递细胞摄取和处理这些免疫复合物,促进肿瘤抗原呈递给T细胞,产生T细胞记忆反应和长期抗肿瘤疫苗效应68。研究发现曲妥珠单抗通过FcγR介导机制增加树突状细胞对HER2抗原的摄取和抗原特异性细胞毒性T细胞生成4。
先天性和适应性免疫共同参与了曲妥珠单抗对乳腺癌的治疗6-7 69。曲妥珠单抗+化疗新辅助治疗HER2+乳腺癌Ⅱ期研究发现,pCR患者NK细胞占比和HER2特异性T细胞增加69。N9831研究曲妥珠单抗+化疗辅助治疗HER2+乳腺癌,相较单独化疗,联合治疗组内源性抗HER2抗体更高,且抗HER2抗体与DFS密切相关7。N0337和N983252研究曲妥珠单抗+化疗治疗转移HER2+乳腺癌发现抗HER2抗体与改善PFS相关6。Fc工程改造的马吉妥昔单抗治疗后T细胞克隆增加,HER2特异性T细胞聚集,HER2特异性抗体水平显著升高70-71。马吉妥昔单抗+帕博利珠单抗治疗HER2+胃癌患者血液样本中同样观察到HER2特异性T细胞增加72。
抗HER2抗体与检查点抑制剂或共刺激剂的组合
最新研究数据显示,大部分HER2+乳腺癌患者,乳腺癌细胞中存在高肿瘤浸润淋巴细胞和PD-L1表达73。另外,曲妥珠单抗治疗会引起HER2+乳腺癌细胞中PD-L1表达上调。已知PD-1/PD-L1上调会导致免疫逃逸。NeoSphere研究发现,PD-1/PD-L1 mRNA高表达与pCR率低相关51。
曲妥珠单抗联合帕博利珠单抗治疗曲妥珠单抗耐药、晚期HER2+乳腺癌(PANACEA)研究发现,PD-L1阳性患者ORR为15%,但PD-L1阴性患者无反应74。另一项曲妥珠单抗+度伐利尤单抗治疗HER2+MBC(IND229)研究,PD-L1阴性患者同样无应答75。
CD137(4-1BB)是一种活化诱导共刺激分子,在活化的T细胞、NK细胞、树突状细胞、嗜酸性粒细胞、肥大细胞、内皮细胞和一些肿瘤细胞上表达76。激动性抗体联合CD137可在多个免疫细胞亚群中提供共刺激信号,包括增强ADCC和ADCP140。CD137激动剂抗体联合曲妥珠单抗或T-DM1治疗难治性HER2+MBC肿瘤的IB/II期临床试验正在进行中。另外特异性双抗(HER2×CD137×CD137)可能有助于进一步评估CD137+抗HER2策略的有效性77。
结论
FcγR依赖性机制是治疗性mAb抗肿瘤作用的重要角色,如(1)影响FcγR结合的Fc结构域糖基化模式的不同抗肿瘤反应,(2)FcγR基因型对曲妥珠单抗治疗HER2 + 乳腺癌的临床疗效的影响,以及(3) 增强与激动型FcγRIIIa结合并减弱与抑制型FcγRIIb结合的Fc工程。目前马吉妥昔单抗已被临床证实安全有效,且被FDA批准用于晚期HER2乳腺癌的治疗。

马吉妥昔单抗是一种人鼠嵌合型的单克隆抗体,其Fab段与曲妥珠单抗相同,因此马吉妥昔单抗与曲妥珠单抗对HER2蛋白和HER2阳性细胞具有相似的结合力,对HER2+肿瘤细胞抗增殖活性类似(见图1)。不同的是,Fc工程蛋白改造,改变Fc结构域中5个氨基酸(L235V/F243L/ R292P/Y300L/P396L),从而使激活型受体FcγRIIIa亲和力增加,抑制型受体FcγRIIb亲和力降低。在所有FcγRIIIa基因型中,马吉妥昔单抗结合亲和力均高于曲妥珠单抗,甚至相较于曲妥珠单抗与FcγRIIIa-158V的亲和力,马吉妥昔单抗结合FcγRIIIa-158F的亲和力更甚一筹37-38。SOPHIA研究证实Fc工程改造的临床活性和可行性63,2020年12月6日FDA批准马吉妥昔单抗联合化疗治疗既往接受过两种或以上抗HER2方案治疗(至少一种用于转移治疗)的HER2阳性转移乳腺癌患者。2021 NCCN V2 乳腺癌指南推荐马吉妥昔单抗联合化疗作为HER2阳性转移性乳腺癌的三线治疗方案(2A)。2022年CSCO BC指南推荐马吉妥昔单抗联合化疗作为晚期HER2+乳腺癌的三线治疗方案(2B)。
相较曲妥珠单抗,曲妥珠单抗联合帕妥珠单抗抗肿瘤活性更强24。体外研究发现,马吉妥昔单抗介导的ADCC效应优于曲妥珠单抗和帕妥珠单抗,相较曲妥珠单抗+帕妥珠单抗,马吉妥昔单抗+帕妥珠单抗NK细胞介导的ADCC效应更强78。马吉妥昔单抗+帕妥珠单抗+化疗vs曲妥珠单抗+帕妥珠单抗+化疗治疗早期HER2+乳腺癌FcγRIIIa-158F携带者的新辅助研究(MARGOT),以pCR以及免疫终点(如肿瘤浸润淋巴细胞率、抗HER2抗体和免疫基因表达谱等)作为研究终点的临床研究正在进行中。目前对于FcγRIIIa基因型、FcγRIIa基因型和FcγRIIb基因型对临床疗效的影响结果不一,但多数研究为回顾性分析,混杂因素太多。MARGOT研究是专门针对FcγRIIIa-158F携带者的前瞻性研究,也许会对FcγRIIIa基因型与临床疗效的关系给出一个直接的答复。
体外研究表明,马吉妥昔单抗治疗后,T细胞克隆增加,HER2特异性T细胞聚集,HER2特异性抗体水平显著升高70-71。目前尚无马吉妥昔单抗适应性免疫应答与曲妥珠单抗适应性免疫应答的头对头比较研究,但对不同研究根据可比分析发现,马吉妥昔单抗适应性免疫应答更优,如循环HER2特异性抗体增加(42%-69%vs94%),T细胞介导反应增加(50%-78%vs98%)71。马吉妥昔单抗+CD137激动剂vs曲妥珠单抗+CD137激动剂治疗HER2+乳腺癌,马吉妥昔单抗+CD137激动剂组是否更优,还有待进一步的探讨,也许是未来研究的新方向。
[1] Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83.
[2] Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92.
[3] Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 2006;94:259–67.
[4] Gall VA, Philips AV, Qiao N, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res 2017;77:5374–83.
[5] Gül N, van Egmond M. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res 2015;75:5008–13.
[6] Knutson KL, Clynes R, Shreeder B, et al. Improved survival of HER2+ breast cancer patients treated with trastuzumab and chemotherapy is associated with host antibody immunity against the HER2 intracellular domain. Cancer Res 2016;76:3702–10.
[7] Norton N, Fox N, McCarl C-A, et al. Generation of HER2-specific antibody immunity during trastuzumab adjuvant therapy associates with reduced relapse in resected HER2 breast cancer. Breast Cancer Res 2018;20:52.
[8] Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res 2007;13:5133–43.
[9] Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity 2006;24:19–28
[10] Vogelpoel LTC, Baeten DLP, de Jong EC, et al. Control of cytokine production by human Fc gamma receptors: implications for pathogen defense and autoimmunity. Front Immunol 2015;6:79
[11] Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008;8:34–47.
[12] Gillis C, Gouel-Chéron A, Jönsson F, et al. Contribution of human FcγRs to disease with evidence from human polymorphisms and transgenic animal studies. Front Immunol 2014;5:254.
[13] Musolino A, Gradishar WJ, Rugo HS,et al. Role of Fcγ receptors in HER2-targeted breast cancer therapy.J Immunother Cancer. 2022 Jan;10(1):e003171.
[14] Teige I, Mårtensson L, Frendéus BL. Targeting the antibody checkpoints to enhance cancer immunotherapy-focus on FcgammaRIIB. Front Immunol 2019;10:481.
[15] Chen X, Song X, Li K, et al. FcgammaR-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol 2019;10:292.
[16] Muntasell A, Cabo M, Servitja S, et al. Interplay between natural killer cells and anti-HER2 antibodies: perspectives for breast cancer immunotherapy. Front Immunol 2017;8:1544.
[17] Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000;6:443–6.
[18] Negri FV, Musolino A, Naldi N, et al. Role of immunoglobulin G fragment C receptor polymorphism-mediated antibody-dependant cellular cytotoxicity in colorectal cancer treated with cetuximab therapy. Pharmacogenomics J 2014;14:14–19.
[19] Robak T, Robak E. New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies. BioDrugs 2011;25:13–25.
[20] Pegram MD, Baly D, Wirth C. Antibody dependent cell-mediated cytotoxicity in breast cancer patients in phase III clinical trials of a humanized anti-HER2 antibody. Proc Am Assoc Cancer Res 1997;38:Abstract 602.
[21] Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128:347–56.
[22] Dokter W, Ubink R, van der Lee M, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther 2014;13:2618–29.
[23] Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107:1039–46
[24] Liu L, Yang Y, Burns R. Margetuximab mediates greater Fcdependent anti-tumor activities than trastuzumab or pertuzumab in vitro. Cancer Res 2019;79:Abstract 1538.
[25] Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330–6.
[26] Swain SM, Miles D, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519–30.
[27] Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791–800.
[28] Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008;8:59–73.
[29] Di Modica M, Sfondrini L, Regondi V, et al. Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2Dmediated NK cell recognition. Oncotarget 2016;7:255–65.
[30] Kim S, Song J, Park S, et al. Drifts in ADCC-related quality attributes of Herceptin®: impact on development of a trastuzumab biosimilar. MAbs 2017;9:704–14.
[31] Pivot X, Pegram M, Cortes J, et al. Three-year follow-up from a phase 3 study of SB3 (a trastuzumab biosimilar) versus reference trastuzumab in the neoadjuvant setting for human epidermal growth factor receptor 2-positive breast cancer. Eur J Cancer 2019;120:1–9.
[32] Pereira NA, Chan KF, Lin PC, et al. The "less-is-more" in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018;10:693–711.
[33] Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003;278:3466–73.
[34] Fiedler W, Stoeger H, Perotti A, et al. Phase I study of TrasGEX, a glyco-optimised anti-HER2 monoclonal antibody, in patients with HER2-positive solid tumours. ESMO Open 2018;3:e000381.
[35] Suzuki E, Niwa R, Saji S, et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin Cancer Res 2007;13:1875–82.
[36] Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell 2018;9:63–73.
[37] Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res 2011;13:R123.
[38] Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res 2007;67:8882–90.
[39] Xiao H, Woods EC, Vukojicic P, et al. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A 2016;113:10304–9.
[40] Maruyama T, Mimura K, Izawa S, et al. Lapatinib enhances herceptin-mediated antibody-dependent cellular cytotoxicity by up-regulation of cell surface HER2 expression. Anticancer Res 2011;31:2999–3005.
[41] Musolino A, Boggiani D, Pellegrino B, et al. Role of innate and adaptive immunity in the efficacy of anti-HER2 monoclonal antibodies for HER2-positive breast cancer. Crit Rev Oncol Hematol 2020;149:102927.
[42] Oberg HH, Kellner C, Gonnermann D, et al. Tribody [(HER2 ) 2 xCD16] is more effective than trastuzumab in enhancing γδ T cell and natural killer cell cytotoxicity against HER2 -expressing cancer cells. Front Immunol 2018;9:814.
[43] Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51.
[44] Grugan KD, McCabe FL, Kinder M, et al. Tumor-associated macrophages promote invasion while retaining Fc-dependent antitumor function. J Immunol 2012;189:5457–66. [45] Yin J, Albers AJ, Smith TS, et al. Differential regulation of human monocytes and NK cells by antibody-opsonized tumors. Cancer Immunol Immunother 2018;67:1239–50.
[46] Shi Y, Fan X, Deng H, et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcgamma receptors on macrophages. J Immunol 2015;194:4379–86.
[47] Herter S, Birk MC, Klein C, et al. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol 2014;192:2252–60.
[48] Richards JO, Karki S, Lazar GA, et al. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008;7:2517–27.
[49] 108 Zhang H, Lu H, Xiang L, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 2015;112:E6215–23.
[50]Tsao L-C, Crosby EJ, Trotter TN. Cd47 blockade augmentation of trastuzumab antitumor efficacy dependent on antibody-dependent cellular phagocytosis. JCI Insight 2019;4:e131882. [51] Upton R, Feng D, Banuelos AM. Humanized anti-CD47 monoclonal antibody magrolimab (Hu5F9-G4) plus trastuzumab potentiates antibody-dependent cellular phagocytosis (ADCP), and cooperate to inhibit human HER2+ breast cancer (BC) xenografts growth in vivo. Abstract PS17-06. Cancer Res 2021;81:PS17–06.
[52] Hu X, Liu Y, Zhang X, et al. The anti-B7-H4 checkpoint synergizes trastuzumab treatment to promote phagocytosis and eradicate breast cancer. Neoplasia 2020;22:539–53.
[53] Laengle J, Kabiljo J, Hunter L. Histone deacetylase inhibitors valproic acid and vorinostat enhance trastuzumab-mediated antibody-dependent cell-mediated phagocytosis. J Immunother Cancer 2020;8:e000195.
[54] Musolino A, Naldi N, Dieci MV, et al. Immunoglobulin G fragment C receptor polymorphisms and efficacy of preoperative chemotherapy plus trastuzumab and lapatinib in HER2-positive breast cancer. Pharmacogenomics J 2016;16:472–7.
[55] Tamura K, Shimizu C, Hojo T, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 2011;22:1302–7.
[56] Botticelli A, Mazzuca F, Borro M, et al. FCGRs polymorphisms and response to trastuzumab in patients with HER2-positive breast cancer: far from predictive value? World J Oncol 2015;6:437–40.
[57] Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673–84.
[58] Gavin PG, Song N, Kim SR, et al. Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2-positive breast cancer: analysis of the NSABP B-31 trial. JAMA Oncol 2017;3:335–41.
[59] Hurvitz SA, Betting DJ, Stern HM, et al. Analysis of Fcgamma receptor IIIA and IIA polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Cancer Res 2012;18:3478–86.
[60] Norton N, Olson RM, Pegram M, et al. Association studies of Fcgamma receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Cancer Immunol Res 2014;2:962–9.
[61] Roca L, Diéras V, Roché H, et al. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCERPACS 04 trial. Breast Cancer Res Treat 2013;139:789–800.
[62] Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008;26:1789–96.
[63] Rugo HS, Im S-A, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021;7:573–84
[64] Tamura K, Shimizu C, Hojo T, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 2011;22:1302–7.
[65] Wang D-S, Wei X-L, Wang Z-Q, et al. FcgammaRIIA and IIIA polymorphisms predict clinical outcome of trastuzumab-treated metastatic gastric cancer. Onco Targets Ther 2017;10:5065–76.
[66] Barnhart BC, Quigley M. Role of Fc-FcgammaR interactions in the antitumor activity of therapeutic antibodies. Immunol Cell Biol 2017;95:340–6.
[67] Ferris RL, Lenz H-J, Trotta AM, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018;63:48–60.
[68] DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell 2015;161:1035–45.
[69] Muraro E, Comaro E, Talamini R, et al. Improved natural killer cell activity and retained anti-tumor CD8(+) T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med 2015;13:204.
[70] S-A I, Bang Y-J, D-Y O. Long-term responders to single-agent margetuximab, an Fc-modified anti-HER2 monoclonal antibody, in metastatic HER2+ breast cancer patients with prior anti-HER2 therapy. Cancer Res 2019;79:Abstract P6-18-11.
[71] Nordstrom JL, Muth J, Erskine CL. High frequency of HER2-specific immunity observed in patients (pts) with HER2+ cancers treated with margetuximab (M), an Fc-enhanced anti-HER2 monoclonal antibody (mAb). J Clin Oncol 2019;37:1030.
[72] Catenacci DVT, Kang Y-K, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a singlearm, phase 1b-2 trial. Lancet Oncol 2020;21:1066–76.
[73] Krasniqi E, Barchiesi G, Pizzuti L, et al. Immunotherapy in HER2- positive breast cancer: state of the art and future perspectives. J Hematol Oncol 2019;12:111.
[74] Loi S, Giobbie-Hurder A, Gombos A, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol 2019;20:371–82.
[75] Chia S, Bedard PL, Hilton J, et al. A phase Ib trial of durvalumab in combination with trastuzumab in HER2-positive metastatic breast cancer (CCTG IND.229). Oncologist 2019;24:1439–45.
[76] Yonezawa A, Dutt S, Chester C, et al. Boosting cancer immunotherapy with anti-CD137 antibody therapy. Clin Cancer Res 2015;21:3113–20
[77] Liu L, Lam C-YK, Alderson R. Selection of a bispecific trivalent HER2 x CD137 trident format providing optimal tumor-anchored immune co-stimulation. Cancer Res 2019;79:1560
[78] Tóth G, Szöőr Árpád, Simon L, et al. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. MAbs 2016;8:1361–70
Expire Date 2023/5/5
ZMCNNP20220425003
排版编辑:肿瘤资讯-张欣明











 苏公网安备32059002004080号
苏公网安备32059002004080号


