点评:林榕波

医学博士,北京大学肿瘤医院日间病房主治医师。
目前主要从事消化道肿瘤及肿瘤姑息治疗。
曾多次参与国内外学术交流,在SCI及国内核心杂志发表多篇论文。
参与编写《肿瘤姑息支持治疗教程》及校对《尊严疗法》书稿等。
额外增加奥氮平到昂丹司琼和地塞米松来预防化疗诱导的恶心呕吐的有效性和安全性:一项随机、双盲、安慰剂对照、交叉研究(ABS:10019)
First Author: Veerisa Vimolchalao, Division of Oncology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
背景
目前对于预防高致吐化疗方案(HEC)诱导的恶心呕吐(CINV)推荐的止吐方案包含地塞米松,帕洛诺司琼联合一种NK-1拮抗剂或奥氮平。然而,帕洛诺司琼和NK-1拮抗剂价格昂贵,并非所有泰国患者可获得。我们试图评估接受HEC患者中额外增加奥氮平到昂丹司琼和地塞米松来预防CINV的有效性和安全性。
方法
入组既往未化疗的患者接受蒽环类-环磷酰胺或高剂量顺铂(≥50mg/m2)方案,随机分配至接受奥氮平或安慰剂加入昂丹司琼和地塞米松。所有受试者在第2周期时交叉至另一组。主要研究终点为完全缓解(CR)率,定义为无呕吐且未使用解救药物。
结果
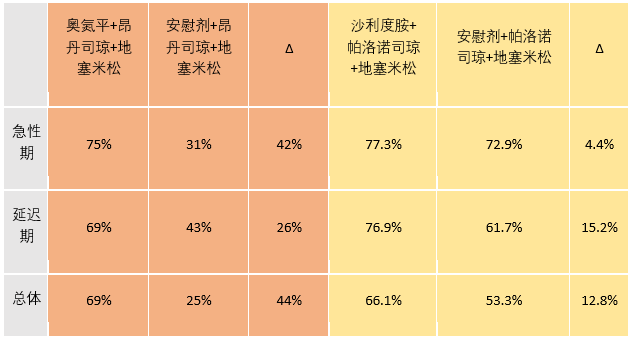
在第1周期,32例接受奥氮平患者的CR率为69%,而32例接受安慰剂患者的CR率为25%,p<0.001。奥氮平对比安慰剂的CR率在急性阶段(0-24h)(75% vs. 31%,p<0.001)和延迟阶段(24-120h)(69% vs. 43%,p=0.038)均有显著改善。应用两个交叉止吐方案后的分析显示,奥氮平组对比安慰剂组在急性阶段(72% vs. 33%, p<0.001),延迟阶段(67% vs. 38%, p< 0.001)和总体阶段(67% vs. 25%,p<0.001)的CR率均改善。使用视觉模拟评分(VAS)进行交叉分析,奥氮平组患者恶心(1.28 vs. 3.05,p<0.001)和疲乏(3.5 vs. 4.58,p<0.001)上有低的平均VAS,而在食欲(2.5 vs. 1.55,p=0.003)和失眠(3.26 vs. 2.2,p<0.001)上有高的平均VAS。没有3-4级止吐药物相关的毒性。平均QT间期改变在两组之间没有显著区别(-4.30ms vs. -1.86ms,p=0.69)。60例患者中52例联合奥氮平对比安慰剂更优(p<0.001)。
结论
没有NK-1拮抗剂时,额外增加奥氮平到昂丹司琼和地塞米松可以显著提高接受HEC患者的CINV预防,并且是安全的。
米氮平预防高致吐化疗方案诱导的延迟性恶心呕吐的有效性:一项开放、随机、多中心III期研究(ABS:1078)
First Author: Jun Cao, Fudan University Shanghai Cancer Center, Shanghai, China
背景
我们研究在接受高致吐化疗方案(HEC)患者中米氮平对预防延迟性恶心呕吐的有效性。
方法
入组接受AC或含有顺铂方案并出现延迟性呕吐的乳腺癌患者,且随后接受至少3周期相同化疗方案的患者,随机分配至米氮平组(每天15mg,第2-4天)或对照组。两组均联合阿瑞匹坦,一种5-HT3受体拮抗剂和地塞米松(7.5mg,第2-4天)。主要研究终点为延迟阶段(25-120h)呕吐的完全缓解(CR)(无呕吐且无解救治疗)。次要研究终点包括急性阶段(0-24h)和总体阶段(0-120h)的CR,和以上三个阶段的完全控制(CC;无呕吐,无解救药物应用,并且仅为1级的恶心)。
结果
本研究2018年1月份由于入组缓慢提前关闭。95例患者中,米氮平组46例,对照组49例。相比对照组,米氮平组第1周期延迟性和总体CR率显著提高:分别为78.3% vs. 49.0%(P=0.003)和58.7% vs. 34.7%(P=0.019)。第3周期结果类似,显示米氮平组延迟性CR率显著提高:分别为88.2% vs. 55.0%(P=0.010)。第1周期和第2周期米氮平组延迟性和总体CC率均显著提高:第1周期分别为:76.1% vs. 49.0%(P=0.006)和56.5% vs. 32.7%(P=0.019);第2周期分别为70.0% vs. 45.7%(P=0.049)和50.0% vs. 25.7% (P=0.043)。在第3周期,奥氮平组延迟性CC率显著升高:88.2% vs. 50.0%(P=0.010)。不良反应轻到中度。米氮平组增加了嗜睡和体重增加。
结论
在出现延迟性呕吐的乳腺癌患者中,使用之前相同的化疗方案时,米氮平联合阿瑞匹坦,一种5-HT3受体拮抗剂和地塞米松显著改善HEC诱导的延迟性恶心呕吐。临床研究编号:NCT02336750。
专家点评

福建省肿瘤医院肿瘤内科副主任医师
福建省抗癌协会癌症康复与姑息治疗专业委员会青年委员会副主任委员
全国肿瘤姑息治疗与人文关怀专业委员会常委
福建省抗癌协会肿瘤内科专业委员会委员
福建省肿瘤转化医学重点实验室主要成员
ASCO会员
2012-2015年援博茨瓦纳公主玛丽娜医院,肿瘤科科主任
今年是我援非回来第三年接受刘巍教授的邀请点评今年ASCO关于CINV方面的研究。虽然今年CINV方面没有特别有影响力的研究,但仍有一些东西值得我们思考。
奥氮平是一种非典型抗精神病药,因可拮抗多种CINV受体(多巴胺、5-羟色胺、组胺和乙酰胆碱毒蕈碱受体)也被用于止吐。 2016年在新英格兰杂志上发表的一项III期研究对比奥氮平与安慰剂联合NK-1 RA(阿瑞匹坦或福沙匹坦)+5-HT3 RA+地塞米松预防HEC患者的CINV。结果显示含奥氮平的4药方案对比安慰剂组(3药方案)显著提高了CR率(无呕吐且无解救治疗)。[1]此后,奥氮平的受关注度明显提高,其组成的3药方案或4方案已经成为多个指南的标准止吐方案。摘要10019是在泰国缺乏NK-1 RA药物的背景下进行的,研究证实在昂丹司琼和地塞米松中加入奥氮平(3药方案)可以显著减少HEC患者的CINV的发生。另一方面,恶心其实是比呕吐更难控制的CINV症状,特别是延迟期。而在奥氮平对比阿瑞匹坦联合帕洛诺司琼和地塞米松的III期研究中,含奥氮平3药组在急性期、延迟期CR率(无呕吐且无解救治疗)以及急性期无恶心率方面与含阿瑞匹坦3药组相似,分别为97% v.s. 87%,77% v.s. 73%,87% v.s. 87%。但含奥氮平3药组在延迟期恶心方面优于阿瑞匹坦3药组(69% v.s. 38%)。[2]而在毒性方面,Spriggs DR教授[3]认为短期使用奥氮平风险很小,镇静是最常见的短期毒性,但通常只是一过性的。体重增加以及锥体外系等症状通常出现在治疗精神病患者长期大剂量奥氮平的情况下。作为止吐药物只是间断性使用,发生这方面的可能性并不大。对于老年人或过度镇静患者奥氮平剂量也可从10mg减为5mg,这并不降低止吐疗效。[4] 因此,当选择3药方案时,考虑到药物经济学,含奥氮平的3药方案是否有它的优势呢?特别在那些经济相对落后的区域。
另一个很有意思的问题就是中国学者在去年JCO上发表的沙利度胺联合帕洛诺司琼和地塞米松对比安慰剂联合帕洛诺司琼和地塞米松可以改善HEC患者延迟期的恶心和呕吐发生率(CLOG1302研究)。[5]将CLOG1302研究和摘要10019研究的第一化疗周期呕吐发生率直接进行对比(见表),含奥氮平3药组比含沙利度胺3药组在急性期、延迟期和总体期改善呕吐发生率绝对值都有较大优势。是因为奥氮平优于沙利度胺,还是因为联合不同5-HT3 RA的原因,还是其他原因?头对头对比奥氮平与沙利度胺也是一件很有趣的事情。
前面提到含奥氮平的4药方案已被指南推荐为HEC方案的一线选择。但是在临床上仍存在赞成和反对的意见。一些持反对意见的学者支持4药方案用在3药常规止吐方案无效之后。[3]摘要10078研究就是米氮平代替奥氮平组成4药联合方案使用在前一周期出现延迟性呕吐的患者上。米氮平是另一个作用在去甲肾上腺素和5-羟色胺受体拮抗剂的非典型抗精神病药。其存在耐受性较好,几乎无抗胆碱能作用,对心血管系统无影响等优点。摘要10078虽然因入组缓慢研究提前关闭,我们还是可以看到含米氮平的4药止吐方案对比对照组可以显著改善在常规3药物方案失败后的HEC患者后续周期的延迟性CINV。另外通过建立风险因素评估来筛选更高危患者一线直接使用含奥氮平的4药方案也是目前研究的方向。[6-8]
表 摘要10019研究(红色)vs CLOG1302研究(黄色)在第一周期HEC的无呕吐率
附摘要原文:
Abstract 10019
Efficacy and safety of additional olanzapine to ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting: A randomized, double-blind, placebo-controlled, crossover study.
First Author: Veerisa Vimolchalao, Division of Oncology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Background: Currently, the antiemetic regimen consisting of dexametha- sone, palonosetron plus a NK-1 antagonist or olanzapine is recommended in prevention of chemotherapy induced nausea and vomiting (CINV) for highly emetogenic chemotherapy (HEC). However, palonosetron and NK-1 antagonists are costly and not accessible for all Thai patients. We sought to evaluate efficacy and safety of the additional olanzapine to ondansetron and dexamethasone for CINV prevention in patients receiving HEC.
Methods: We randomly assigned chemotherapy-na ̈ıve patients receiving either anthracycline- cyclophosphamide or high dose cisplatin ($50 mg/m2) regimen, to receive olanzapine or placebo in addition to ondansetron and dexamethasone. All subjects were crossed over to another arm on second-cycle chemotherapy. The primary endpoint was complete response (CR) rate defined as no vomiting and no use of rescue drugs.
Results: At first cycle, CR was 69% among the 32 patients receiving olanzapine and 25% among the 32 patients receiving placebo, p <0.001. CR was significantly better with olanzapine than with placebo in acute phase (0-24 h) (75% vs. 31%, p <0.001) and delayed phase (24-120 h) (69% vs. 43%, p = 0.038). In analysis after two crossover antiemetic regimens, CR was improved in olanzapine group compared to placebo group in acute phase (72% vs. 33%, p <0.001), delayed phase (67% vs. 38%, p <0.001) and overall period (67% vs. 25, p <0.001). In crossover analysis using visual analog score (VAS), the patients with olanzapine had lower mean VAS in nausea (1.28 vs. 3.05, p , 0.001) and fatigue (3.5 vs. 4.58, p <0.001) but higher mean VAS in appetite (2.5 vs. 1.55, p = 0.003) and sleepiness (3.26 vs. 2.2, p<0.001). There were no grade 3 and 4 antiemetic-drug-related toxicities. Mean QT interval change did not different between two groups (-4.30 ms vs. -1.86 ms, p = 0.69). The olanzapine combination was preferable to placebo in 52 of 60 patients (p<0.001).
Conclusions: WithouttheNK-1antagonists,theadditionalolanzapineto ondansetron and dexamethasone significantly improved CINV prevention and was safe in patients receiving HEC.
Abstract 1078
Efficacy of mirtazapine in preventing delayed nausea and vomiting induced by highly emetogenic chemotherapy: An open-label, randomized, multicenter phase III trial.
First Author: Jun Cao, Fudan University Shanghai Cancer Center, Shanghai, China
Background: We examined the efficacy of mirtazapine for the prevention of delayed nausea and vomiting in patients who received highly emetogenic chemotherapy (HEC).
Methods: Patients with breast cancer who experi- enced delayed emesis after receiving AC or cisplatin containing regimens, and would subsequently accept at least 3 cycles of the same chemotherapy were randomly assigned to a mirtazapine group (15 mg daily on days 2 to 4) or control group, both with aprepitant, a 5-HT3 receptor antagonist and dexamethasone (7.5 mg on day 2 to 4). Primary end point was complete response (CR) to vomiting (no emesis and no rescue treatments) in the delayed phase (25 to 120 h). Secondary end points included CR during acute (0 to 24 h) and overall (0 to 120 h) periods, complete control (CC) (no emesis, no rescue medication use, and no more than grade 1 nausea) during the 3 periods above.
Results: The study was closed early in January, 2018 due to the slow enrollment. Of 95 patients, 46 in the mirtazapine group and 49 in the control group. Compared with control group in the 1st cycle, delayed and overall CR rates were significantly higher with mirtazapine: 78.3% versus 49.0% (P = 0.003) and 58.7% versus 34.7% (P = 0.019), respectively. Similar result was observed in the 3rd cycle, which showed that delayed CR rates was significantly higher with mirtazapine: 88.2% versus 55.0%, respectively (P = 0.010). Delayed and overall CC rates were sig- nificantly higher with mirtazapine both in the 1st and 2nd cycles: 76.1% versus 49.0% (P = 0.006) and 56.5% versus 32.7% (P = 0.019), re- spectively in the 1st cycle and 70.0% versus 45.7% (P = 0.049) and 50.0% versus 25.7% (P = 0.043), respectively in the 2nd cycle. In the 3rd cycle, delayed CC rates was significantly higher with mirtazapine: 88.2% versus 50.0% (P = 0.010). Adverse effects were mild to moderate. The mirtazapine group had increased somnolence and weight gain.
Conclusions: Mirtazapine with aprepitant, a 5-HT3 receptor antagonist and dexamethasone signifi- cantly improved HEC-induced delayed nausea and vomiting prevention in patients with breast cancer who experienced delayed emesis in the same chemotherapy previously. Clinical trial information: NCT02336750.
1. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2016;375(2):134-42.
2. Navari RM1, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188-95.
3. Wilbur MB, Birrer MJ, Spriggs DR. CLINICAL DECISIONS. Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2016;375(2):177-9.
4. Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. 2018;23(2):382-388.
5. Zhang L, Qu X, Teng Y, et al. Efficacy of Thalidomide in Preventing Delayed Nausea and Vomiting Induced by Highly Emetogenic Chemotherapy: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Phase III Trial (CLOG1302 study). J Clin Oncol. 2017;35(31):3558-3565.
6. Clemons M, Bouganim N, Smith S, et al. Risk Model-Guided Antiemetic Prophylaxis vs Physician's Choice in Patients Receiving Chemotherapy for Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016;2(2):225-31.
7. Dranitsaris G, Molassiotis A, Clemons M, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28(6):1260-1267.
8. Scotté F. Identifying predictive factors of chemotherapy-induced nausea and vomiting (CINV): a novel approach. Ann Oncol. 2017;28(6):1165-1167.















 苏公网安备32059002004080号
苏公网安备32059002004080号


