重磅新闻:
(2017年5月23日,FDA)美国FDA今日加速审批通过了默沙东(MSD)的KEYTRUDA(pembrolizumab)针对特定Biomarker来进行肿瘤患者治疗,这是第一次基于Biomarker而非如同既往基于肿瘤来源的获批。这将是肿瘤药物获批历史上又一个重要的Milestone。
KEYTRUDA(pembrolizumab)获批治疗带有MSI-H(microsatelliteinstability-high,微卫星不稳定性高)或dMMR(mismatch repair deficient,错配修复缺陷)的实体瘤患者,他们的病情在先前的治疗后都出现了进展。
什么是MSI和MMR?
微卫星不稳定性(microsatellite instability,MSI)是指 DNA甲基化或者基因突变致错配修复基因缺失,从而导致微卫星重复序列插入或缺失,继而长度改变,与肿瘤的发生密切相关。根据微卫星的数据分析可有3种结果:高微卫星不稳定性(MSI-H)、低微卫星不稳定性(MSI-L)和微卫星稳定(MSS)。在临床上比较多用于结直肠癌的预后和化疗获益预测,还可以诊断Lynch综合征。
错配修复缺陷是在遗传性非息肉性大肠癌(HNPPC)中分离到的一组遗传易感基因,该系统任一基因突变,都会导致细胞错配修复功能缺陷,结果产生遗传不稳定,表现为复制错误或微卫星不稳定,因而容易发生肿瘤。主要的DNA MMR基因包括MLH1、MSH2、MSH6、PMS2和PMS1。
MSI-H与dMMR是两种常见的遗传异常,含有这两种变异的肿瘤,细胞内的DNA修复机制往往会受到影响,不能正常发挥作用。带有这些异常的肿瘤分布非常广泛,除了肠癌、还可出现于子宫内膜、胃肠道、乳腺、前列腺、膀胱、甲状腺等多个部位。
精准治疗
“异病同治,同病异治”可能是未来肿瘤治疗的方向,越来越多靶点的发现及药物的研发,使得精准医疗进入了新的时代。基于二代测序技术,相应的“雨伞研究”(Umbrella Trial)与篮子研究(BasketTrial)也是现在非常重要的研究方向。与以往按照肿瘤发病器官来区分不同,篮子研究的治疗选择基于某种分子变化。
KEYTRUDA本次获批根据Biomarker的治疗,将是肿瘤治疗的一大步。我们可以预见不远的将来,精准治疗将比预期更快的到来。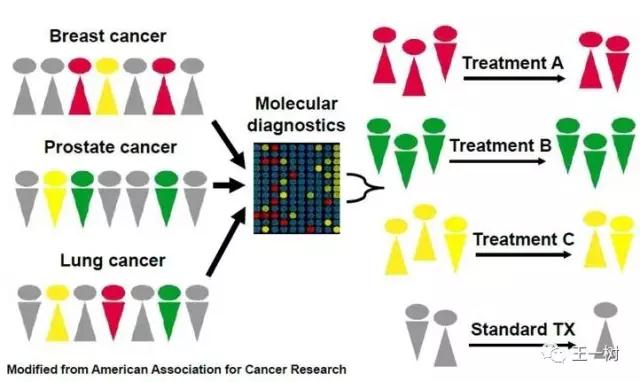
REF:
FDA approves first cancer treatment for any solid tumor with a specificgenetic feature
The U.S. Food and Drug Administration today granted accelerated approvalto a treatment for patients whose cancers have a specific genetic feature(biomarker). This is the first time the agency has approved a cancer treatmentbased on a common biomarker rather than the location in the body where the tumororiginated.
Keytruda (pembrolizumab) is indicated for the treatment of adult andpediatric patients with unresectable or metastatic solid tumors that have beenidentified as having a biomarker referred to as microsatellite instability-high(MSI-H) or mismatch repair deficient (dMMR). This indication covers patientswith solid tumors that have progressed following prior treatment and who haveno satisfactory alternative treatment options and patients with colorectalcancer that has progressed following treatment with certain chemotherapy drugs.
“This is an important first for the cancer community,” said RichardPazdur, M.D., acting director of the Office of Hematology and Oncology Productsin the FDA’s Center for Drug Evaluation and Research and director of the FDA’sOncology Center of Excellence. “Until now, the FDA has approved cancertreatments based on where in the body the cancer started—for example, lung orbreast cancers. We have now approved a drug based on a tumor’s biomarkerwithout regard to the tumor’s original location.”
MSI-H and dMMR tumors contain abnormalities that affect the properrepair of DNA inside the cell. Tumors with these biomarkers are most commonlyfound in colorectal, endometrial and gastrointestinal cancers, but also lesscommonly appear in cancers arising in the breast, prostate, bladder, thyroidgland and other places. Approximately 5 percent of patients with metastaticcolorectal cancer have MSI-H or dMMR tumors.
Keytruda works by targeting the cellular pathway known as PD-1/PD-L1(proteins found on the body’s immune cells and some cancer cells). By blockingthis pathway, Keytruda may help the body’s immune system fight the cancercells. The FDA previously approved Keytruda for the treatment of certainpatients with metastatic melanoma, metastatic non-small cell lung cancer,recurrent or metastatic head and neck cancer, refractory classical Hodgkinlymphoma, and urothelial carcinoma.
Keytruda was approved for this new indication using theAcceleratedApprovalpathway, under which the FDA may approve drugs for serious conditionswhere there is unmet medical need and a drug is shown to have certain effectsthat are reasonably likely to predict a clinical benefit to patients. Furtherstudy is required to verify and describe anticipated clinical benefits ofKeytruda, and the sponsor is currently conducting these studies in additionalpatients with MSI-H or dMMR tumors.
The safety and efficacy of Keytruda for this indication were studied inpatients with MSI-H or dMMR solid tumors enrolled in one of five uncontrolled,single-arm clinical trials. In some trials, patients were required to haveMSI-H or dMMR cancers, while in other trials, a subgroup of patients wereidentified as having MSI-H or dMMR cancers by testing tumor samples after treatmentbegan. A total of 15 cancer types were identified among 149 patients enrolledacross these five clinical trials. The most common cancers were colorectal,endometrial and other gastrointestinal cancers. The review of Keytruda for thisindication was based on the percentage of patients who experienced complete orpartial shrinkage of their tumors (overall response rate) and for how long(durability of response). Of the 149 patients who received Keytruda in thetrials, 39.6 percent had a complete or partial response. For 78 percent ofthose patients, the response lasted for six months or more.
Common side effects of Keytruda include fatigue, itchy skin (pruritus),diarrhea, decreased appetite, rash, fever (pyrexia), cough, difficultybreathing (dyspnea), musculoskeletal pain, constipation and nausea. Keytrudacan cause serious conditions known as immune-mediated side effects, includinginflammation of healthy organs such as the lungs (pneumonitis), colon(colitis), liver (hepatitis), endocrine glands (endocrinopathies) and kidneys(nephritis). Complications or death related to allogeneic hematopoietic stemcell transplantation after using Keytruda has occurred.
Patients who experience severe or life-threatening infusion-relatedreactions should stop taking Keytruda. Women who are pregnant or breastfeedingshould not take Keytruda because it may cause harm to a developing fetus ornewborn baby. The safety and effectiveness of Keytruda in pediatric patientswith MSI-H central nervous system cancers have not been established.
The FDA granted this applicationPriority Reviewdesignation, under whichthe FDA’s goal is to take action on an application within six months where theagency determines that the drug, if approved, would significantly improve thesafety or effectiveness of treating, diagnosing or preventing a seriouscondition.
The FDA granted accelerated approval of Keytruda to Merck & Co.
The FDA, an agency within the U.S. Department of Health and HumanServices, protects the public health by assuring the safety, effectiveness, andsecurity of human and veterinary drugs, vaccines and other biological productsfor human use, and medical devices. The agency also is responsible for thesafety and security of our nation’s food supply, cosmetics, dietary supplements,products that give off electronic radiation, and for regulating tobaccoproducts











 苏公网安备32059002004080号
苏公网安备32059002004080号


