Highlights
•This study, by far the largest to date of dosimetric predictors of RP in the immunotherapy era, illustrates that MLD is the most critical dose-volume parameter influencing RP risk.
•Grades 1–5 RP occurred in 21.9%, 25.0%, 8.3%, 1.6%, and 1.0%, respectively.
•The mean MLD for patients with grades 1–5 RP was 10.7, 11.6, 12.6, 14.7, and 12.8 Gy, respectively.
•On multivariable analysis, tumor location and mean lung dose (MLD) significantly predicted for any-grade, grade ≥ 2. Only MLD significantly predicted for grade ≥ 3 RP.
•It suggested that the well-recognized dose-volume constraints for thoracic RT may require revision in the future if used in the context of prior ICIs.
Abstract
Background and purpose
Combining immune checkpoint inhibitors (ICIs) and thoracic radiotherapy (TRT) may magnify the radiation pneumonitis (RP) risk. Dosimetric parameters can predict RP, but dosimetric data in context of immunotherapy are very scarce. To address this knowledge gap, we performed a large multicenter investigation to identify dosimetric predictors of RP in this under-studied population.
Materials and methods
All lung cancer patients from five institutions who underwent conventionally-fractionated thoracic intensity-modulated radiotherapy with prior ICI receipt were retrospectively compiled. RP was defined per CTCAE v5.0. Statistics utilized logistic regression modeling and receiver operating characteristic (ROC) analysis.
Results
The vast majority of the 192 patients (median follow-up 14.7 months) had non-small cell lung cancer, received PD-1 inhibitors, and did not receive concurrent systemic therapy with TRT. Grades 1–5 RP occurred in 21.9%, 25.0%, 8.3%, 1.6%, and 1.0%, respectively. The mean MLD for patients with grades 1–5 RP was 10.7, 11.6, 12.6, 14.7, and 12.8 Gy, respectively. On multivariable analysis, tumor location and mean lung dose (MLD) significantly predicted for any-grade and grade ≥ 2 pneumonitis. Only MLD significantly predicted for grade ≥ 3 RP. ROC analysis was able to pictorially model RP risk probabilities for a variety of MLD thresholds, which can be an assistive tool during TRT treatment planning.
Conclusion
This study, by far the largest to date of dosimetric predictors of RP in the immunotherapy era, illustrates that MLD is the most critical dose-volume parameter influencing RP risk. These data may provide a basis for revising lung dose constraints in efforts to better prevent RP in this rapidly expanding ICI/TRT population.
Keywords
Radiotherapy;Immune checkpoint;inhibitor;Pneumonitis;Dosimetry;Risk factors
Introduction
The advent of immune checkpoint inhibitors (ICIs) has transformed cancer treatment owing to remarkable efficacy in increasing disease control, overall survival, and improving the quality of life of patients across multiple cancers [1], [2], [3]. Radiotherapy (RT) has long been a cornerstone of cancer treatment, and several studies have demonstrated the potential synergy of combining RT with immunotherapy to control or eradicate cancer [2], [4], [5], [6].
However, ICIs can also induce immune-related adverse events (irAEs) in some patients [7], [8], [9], [10], and ICI-related pneumonitis is a potentially fatal complication in non-small-cell lung cancer (NSCLC) [10], [11], [12]. The combination of ICIs and RT could also increase the risk of radiation pneumonitis (RP) [13], [14]. Traditionally, the radiation dosimetric parameters that have best predicted the occurrence of pneumonitis are the volume of lungs receiving ≥ 20 Gy (V20) [15], [16], the volume of lungs receiving ≥ 5 Gy (V5) [17], and the mean lung dose (MLD) [18], [19]. However, because these parameters were elucidated prior to the immunotherapy era, their accuracy in the context of ICI therapy remains uncertain.
In a previous single-center study of 40 patients [20], 65% of patients who received ICIs before thoracic radiotherapy (TRT) developed acute RP, 40% of whom had symptomatic grade ≥ 2 acute RP. However, that study had some limitations, including a small sample size, short follow-up, and heterogeneous RT techniques/schemes. In order to address those shortcomings, we conducted this multicenter analysis to better identify risk factors and predictors of RP in patients with NSCLC who received ICIs before conventionally-fractionated thoracic intensity-modulated radiotherapy (thoracic RT), which could inform the development of more effective radiation dosimetry parameters for lung cancer patients in the immunotherapy era going forward.
Methods and materials
Patients and treatment
The patient population of this retrospective investigation was lung cancer patients who were treated with PD-1/PD-L1 inhibitors prior to thoracic RT between March 2019 and September 2022 at five hospitals (Hubei Cancer Hospital, Union Hospital, Tongji Hospital, the First Affiliated Hospital of Yangtze University, and Cancer Hospital of Chinese Academy of Medical Science). This study was performed in accordance with the Declaration of Helsinki and approved by the institutional ethics board of Hubei Cancer Hospital of Huazhong University of Science and Technology (LLHBCH2021YN-035) and four other institutions.
The inclusion criteria required all patients to have received both immunotherapy and thoracic RT, have undergone at least one baseline chest computed tomography (CT) scan and at least one follow-up lung CT after RT initiation, and were treated with thoracic RT according to the dose constraints set forth by QUANTEC [15]. Hypofractionated radiotherapy and SBRT patients, as well as those who did not receive immunotherapy before RT or who did not have baseline or follow-up CT scan(s), were excluded. There was no exclusion for histology (NSCLC or small cell lung cancer) or concurrent systemic therapy.
RT was planned and conducted according to the fundamental principles of each particular clinical circumstance at the discretion of the treating physician/institution, and image guidance was used for all cases thrice per week in the first week and twice per week in the remaining weeks. Given the multi-institutional nature of this work, imaging follow-up could not be standardized, but generally consisted of follow-up chest CT (or whole body PET-CT) one month following RT and every 3 months subsequently.
Toxicity assessments
At all centers, the diagnosis of RP was established by a multidisciplinary oncologic team consisting of at least one pulmonologist, one radiation oncologist, and one radiologist. The diagnosis was based on the presence of diffuse lung abnormalities or fibrosis limited or most to the radiation field of the lung on CT scans and clinical symptoms, while excluding other possible causes, such as infection or checkpoint inhibitor-related pneumonitis (CIP). Fig. 1 shows representative CT images of RP, CIP and infection. The severity of RP was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 grading system.
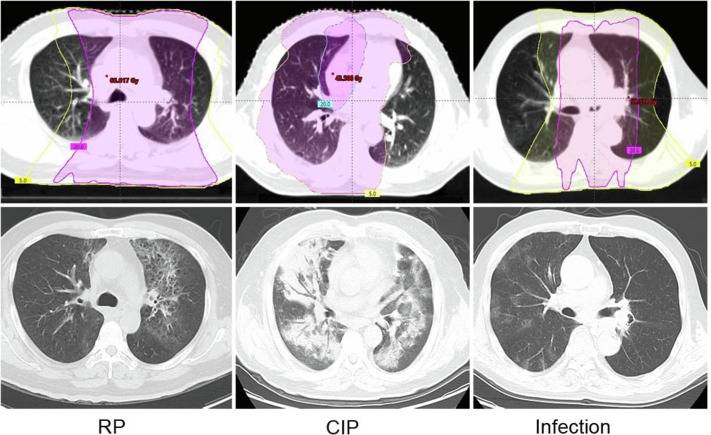
Fig. 1. CT images in patients with pneumonitis.
Statistical analysis
The statistical methods used in this study were patterned after previously published work [20]. We used logistic regression models to evaluate the association between patient characteristics and the risk of RP. We also used the area under the receiver operating characteristic (ROC) curve (AUC) to assess the models' discriminative ability. All data analyses were performed using R software (version 4.1.0) and used 2-sided tests with a p-value less than 0.05 to indicate statistical significance.
Results
A total of 230 patients with lung cancer who had undergone ICIs prior to TRT were screened for this study (Supplemental Table S1). Nine patients who underwent SBRT and 29 who received hypofractionated RT were excluded, leaving a total of 192 patients who received thoracic RT for inclusion (Table 1). Of note, most patients had NSCLC (80.2%) and received PD-1 inhibitors prior to TRT (89.1%). The median number of previous ICI cycles was 4 (IQR: 2–6), and the median time between the final ICI cycle and the start of thoracic RT was 34 days (IQR: 22–49). During thoracic RT, a minority of patients (n = 52, 27.1%) underwent concurrent systemic therapy, comprising 32 (16.7%) who received chemotherapy, 12 (6.3%) who received concurrent ICIs and chemotherapy, 5 (2.6%) who received ICIs monotherapy, and 3 (1.6%) who received other targeted agents. A total of 82 patients received ICI therapy after RT, including 31.8% (42/132) of patients with stage III and 75.5% (40/53) of patients with stage IV disease.
Table 1. Patient Characteristics.
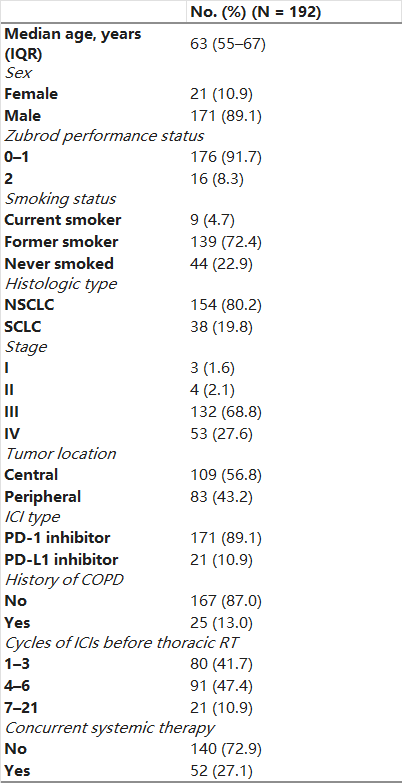
Abbreviations: IQR: Interquartile range, CIs: Immune checkpoint inhibitors; PD-1: programmed death 1; PD-L1: programmed death ligand 1; COPD: chronic obstructive pulmonary disease; thoracic RT: conventional fractionation thoracic intensity-modulated radiotherapy.
Table 2 displays the RT-related characteristics of the study population. RT was delivered at 1.8–2.2 Gy fractions once daily, and the median EQD2 for all patients was 60 Gy (IQR: 58–60). The median (IQR) V20, V5, and MLD were 20% (16.3–23.6), 38% (30.4–47.1), and 11 Gy (8.8–13.3), respectively.
Table 2. Thoracic Radiotherapy Characteristics.
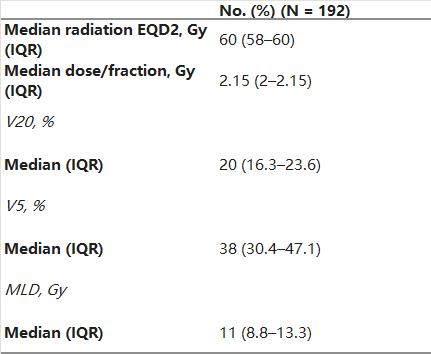
Abbreviations: EQD2, equivalent dose in 2 Gy fractions; IQR: interquartile range; V20: volume of lung receiving ≥ 20 Gy; V5: volume of lung receiving ≥ 5 Gy; MLD: mean lung dose; ICIs: immune checkpoint inhibitors.
The median follow-up time after initiation of thoracic RT was 14.7 months (IQR: 9.0–22.8). Of the 192 patients, 111 (57.8%) experienced any-grade RP, and grades 1–5 RP occurred in 21.9%, 25.0%, 8.3%, 1.6%, and 1.0% of patients, respectively. The median time from the start of thoracic RT to onset of RP was 73 days (IQR: 56–124). Of the 69 patients who developed symptomatic RP, 94.2% (66/69) did so within 6 months (median: 66 days; IQR: 52–90) after the initiation of thoracic RT. Six patients developed symptomatic RP leading to termination of RT during the course of treatment. Non-productive cough (n = 57) was the most common symptom of RP, followed by fever (n = 43) and wheezing (n = 21).
Of the 61 patients (88.4%) treated with systemic steroids, 52 (75.4%) received pulse methylprednisolone intravenously and oral tapering with prednisolone, and the remaining 9 (13%) were treated with oral prednisolone. The median of treatment course was 34 days (IQR: 21–72). Twenty-two (31.9%) patients had persistent RP symptoms (cough) six months from the onset of RP symptoms.
Table 3 presents the univariable analysis of factors associated with RP risk. The only variable that showed a significant association with any-grade RP, grade ≥ 2 RP, and grade ≥ 3 RP was tumor location (p = 0.0022, p = 0.0003, and p = 0.0023, respectively) and MLD (p = 0.0022, p = 0.0003, and p = 0.0023, respectively). V20 was only associated with grade ≥ 3 RP (p = 0.0235). We then conducted multivariable logistic regression analysis (Table 4) and found that MLD and tumor location were significantly associated with the occurrence of any-grade RP (OR 1.33, 95% CI 1.12–1.58, p = 0.0009; OR 3.03, 95% CI 1.57–5.85, p = 0.001, respectively), grade ≥ 2 RP (OR 1.50, 95% CI 1.24–1.82, p<0.0001; OR 4.11, 95% CI 1.94–8.67, p = 0.0002, respectively), and only MLD was significantly associated with the occurrence of grade ≥ 3 RP (OR 1.33, 95% CI 1.04–1.70, p = 0.0235). Age, sex, smoking history, history of COPD, the number of prior ICI cycles, the timing between the final ICI dose and the start of RT, PTV size, and the delivery of concurrent systemic therapy were not significantly associated with RP risk.
Table 3. Univariable analysis of factors associated with RP.
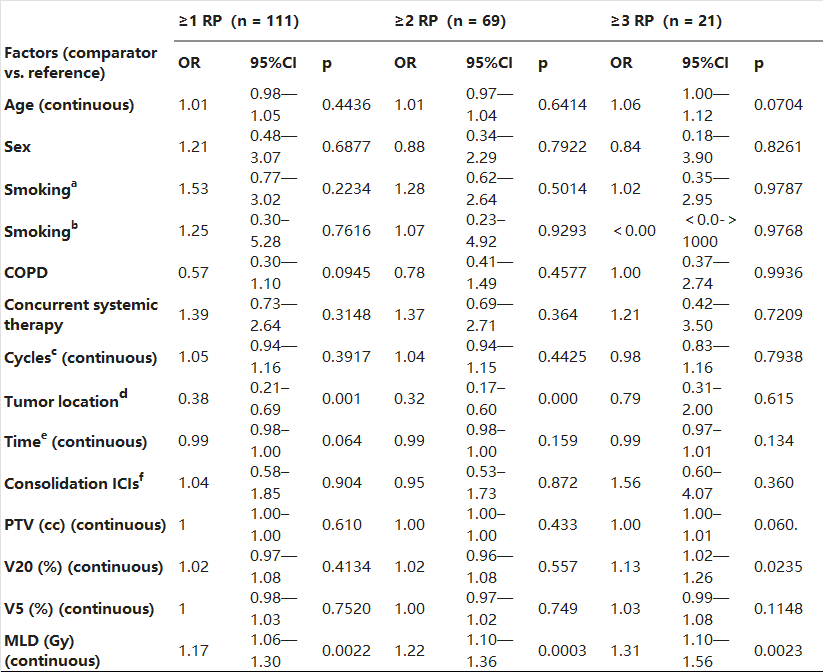
Abbreviations: a: never somked vs. former somker; b: never somked vs. current smoker; c: Cycles of ICI before thoracic RT. d: peripheral vs. central; e: the timing between last dose of ICI and start of thoracic RT; f: Consolidation ICIs after thoracic RT; PTV: planning target volume; V20: volume of lung receiving ≥ 20 Gy; V5: volume of lung receiving ≥ 5 Gy; MLD: mean lung dose; ICIs: immune checkpoint inhibitors; COPD: chronic obstructive pulmonary disease; thoracic RT: conventional fractionation thoracic intensity-modulated radiotherapy.
Table 4. Multivariable analysis of factors related to RP.
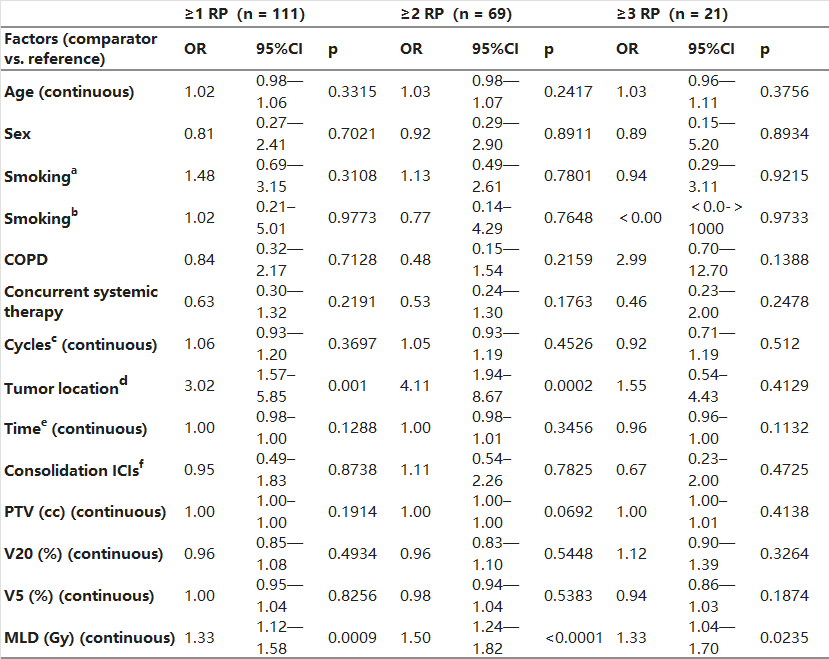
Abbreviations: a: never somked vs. former somker; b: never somked vs. current smoker; c: Cycles of ICI before thoracic RT. d: peripheral vs. central; e: the timing between last dose of ICI and start of thoracic RT; f: Consolidation ICIs after thoracic RT; PTV: planning target volume; V20: volume of lung receiving ≥ 20 Gy; V5: volume of lung receiving ≥ 5 Gy; MLD: mean lung dose; ICIs: immune checkpoint inhibitors; COPD: chronic obstructive pulmonary disease; thoracic RT: conventional fractionation thoracic intensity-modulated radiotherapy.
Fig. 2 depicts the relationship between MLD and RP grade. The mean MLD values by RP grade were as follows: Grade 0, 10.1 Gy; Grade 1, 10.7 Gy; Grade 2, 11.6 Gy; Grade 3, 12.6 Gy; Grade 4, 14.7 Gy; and Grade 5, 12.8 Gy. ROC analysis showed that MLD was associated with grade ≥ 1 RP (AUC = 0.624), grade ≥ 2 RP (AUC = 0.662), and grade ≥ 3 RP (AUC = 0.710). Based on these models, we constructed graphs to depict the incrementally increasing risk of RP based on MLD as a continuous variable (Fig. 3). According to these models, MLD values of 8.8 Gy, 14.1 Gy, and 19.7 Gy would each predict a 50% risk of grade ≥ 1, ≥2, and ≥ 3 RP, respectively (Fig. 3).
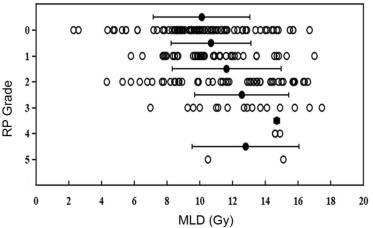 Fig. 2. Relationship between MLD and RP grade.
Fig. 2. Relationship between MLD and RP grade.
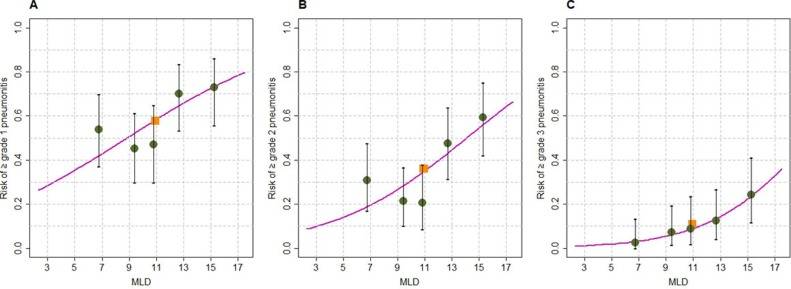 Fig. 3. The risk of RP based on dose-volume parameters. Of note, the y-intercept (corresponding to MLD of 0) is not 0 because immune checkpoint inhibitors are associated with an independent risk of RP.
Fig. 3. The risk of RP based on dose-volume parameters. Of note, the y-intercept (corresponding to MLD of 0) is not 0 because immune checkpoint inhibitors are associated with an independent risk of RP.
Discussion
Despite the promise shown by combining RT and immunotherapy to treat lung cancer, very few studies have explored dosimetric predictors of RP in context of ICI therapy. This multicenter study, by far the largest such study to date, indicates that MLD may be the most important dose-volume parameter that should be addressed during treatment planning for these patients. These data also provide a compelling rationale to revise tolerance thresholds and planning objectives when used in context of immunotherapy.
This study takes a considerable step forward in efforts to discern dose-volume thresholds for the lung in patients undergoing combined RT and immunotherapy. Unfortunately, small sample sizes have largely hampered such efforts in the past. This multicenter study encompassed a higher sample size than the three previous studies put together; the much higher number of statistical events (i.e., RP occurrences) allow for adequate statistical discernment of dosimetric parameters associated with RP, including with multivariable adjustment (which has not been conducted before on account of low sample sizes and event rates). The multivariable analysis results herein provide the most statistically robust evidence that MLD is the most important parameter associated with RP in this population.
Our study findings suggest that revision of the accepted MLD tolerance limits in patients receiving prior ICIs is the next major step. The QUANTEC RP model (published well before the utilization of immunotherapy) indicates that symptomatic RP risk is less than 20% when MLD is no more than 20 Gy [21], which is very different from estimates derived from the ICI-TRT population herein (roughly 7 Gy). Although such dose-volume constraints are difficult to achieve in clinical practice, and individualized constraints may be most optimal until prospective data corroborate the findings herein, we do encourage that clinicians should use some degree of stricter MLD constraints (or more carefully implementing the ALARA principle) in patients receiving combined ICIs and TRT than those posited by QUANTEC. To this end, we have designed a prospective observational trial to corroborate these results (NCT05219851) as well as a single-arm, open-label, phase II trial of pirfenidone to address RP in this population (NCT05801133).
The multi-institutional nature of this work is better reflective of “real-world” treatment patterns, which could be a prime reason why the incidence of high-grade (≥3) RP was considerably lower in this study (11%) than previously described (22.5%) [20]. In prior work, V20 was associated with grade ≥ 2 RP, but that was based on univariable analysis only and was not corroborated by larger sample sizes herein. Additionally, the aforementioned publication had shown that a MLD of 5.55 Gy would predict for a 50% risk of grade ≥ 1 RP, which was further refined herein (8.8 Gy). Finally, the modeling curves in Fig. 3 qualitatively show considerably less heterogeneity than those of the previous investigation, with particular emphasis on the consistency and fit for the grade ≥ 3 RP curve herein.
This results in our study showed that centrally-located disease was significantly associated with the occurrence of grade ≥ 2 RP, which is consistent with prior work [22]. This may be attributed to the treatment of tumors in the center of the lung often resulting in constriction or occlusion of the major bronchi, which makes the lungs more vulnerable to radiation-induced toxicity [23]. Omission of the clinical target volume (CTV) may be a strategy that could reduce the radiation dose to normal lung tissues [24], [25], but remains largely unproven. The clinical implications of these findings offer insights into the management of patients with a central tumor undergoing thoracic RT combined with ICI treatment.
Given the longer follow-up of this study as compared to prior data, it can be noted that 94.2% of patients with symptomatic RP developed symptoms within 6 months (median time 66 days; IQR: 52–90) after the initiation of thoracic RT. Six patients developed symptomatic RP (with fever, cough, and wheezing) during thoracic RT, and all patients discontinued treatment. Therefore, these cases suggest that the incidence of RP may be higher and occur earlier in patients receiving thoracic RT after the initiation of immunotherapy. Close monitoring should be recommended in this patient population during thoracic RT and follow-up, even in the early period of thoracic RT.
These data are likely to become considerably more applicable going forward, as the use of combined RT and immunotherapy is rapidly increasing, with multiple positive randomized trials as well as numerous ongoing trials [26]. These data of ICI therapy followed by thoracic RT are most applicable to contemporary paradigms of oligometastatic or polymetastatic NSCLC (e.g. the ongoing randomized NRG LU002, SARON, and SABR-COMET-10 trials), all of which involve delivering first-line immunotherapy followed by RT to the primary thoracic lesion as well as metastatic areas. Our data suggest that potential revisions in lung dose constraints for these patients may prove to be beneficial, especially in light of a recent meta-analysis that illustrated a higher rate of radiation pneumonitis with ICIs followed by thoracic RT (compared to vice-versa or concurrent therapy) [27].
Despite the strengths of this study (e.g., large sample sizes, longer follow-up, relatively similar dose-fractionation schemes, and uniform use of IMRT), several limitations must be acknowledged. First, in addition to retrospective biases, the multi-center nature may have added an element of heterogeneity in the population (e.g. frequency of imaging follow-up can differ by institution). Second, our study primarily involved PD-1 inhibitors, which may have a somewhat modestly higher incidence of pneumonitis compared to PD-L1 inhibitors (3.6% vs. 1.3% for any grade; 1.1% vs. 0.4% for grade ≥ 3) [28], and hence the drug selection bias in our study must be considered. Third, some patients received consolidation immunotherapy in our study. Because ICIs can potentially cause RRP [29], [30], those patients may have a different RP-related risk. Fourth, the AUC values from the ROC analysis in this study were relatively low, indicating that heterogeneity was still clearly present in this dataset. Lastly, we did not evaluate baseline pulmonary function or other adverse events from pre-RT ICI therapy, all of which are candidate variables that may be also associated with RP. It is also acknowledged that these data may not necessarily apply to patients who do not receive ICIs prior to RT (e.g. stage III NSCLC cases treated using the PACIFIC trial paradigm).
Conclusions
This study is by far the largest to date of dosimetric predictors of RP in the immunotherapy era. From these data, MLD is the most critical dose-volume parameter influencing RP risk. These data may provide a basis for revising lung dose constraints in efforts to better prevent RP in this rapidly expanding ICI/TRT population, but further prospective confirmation of these results is necessary.
Funding information
This work was supported by Research Projects of Biomedical Center of Hubei Cancer Hospital (grant number 2022SWZX17) and National Cancer Center Climbing Foundation (grant number NCC201917B03), and Hubei Provincial Health Commission (grant number ZY2021M008), and Open Research Fund of Hubei Key Laboratory of Precision Radiation Oncology (grant number jzfs023).
Author’s Contributions
JP B, GH and VV performed the literature search and study design. JP B, RM, DQ Y, YL, JC, LZ, JQ, XDX SQ H, NB and ZL Y collected the data, processed statistical data, performed and data interpretation. JP B, NB, GH and VV drafted the manuscript. GH, NB and VV revised the final manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
.Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary data 1.
[1]
M.C. Garassino, S. Gadgeel, G. Speranza, E. Felip, E. Esteban, M. Domine, et al.
Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study
J. Clin. Oncol. (2023), p. JCO2201989
[2]
D.R. Spigel, C. Faivre-Finn, J.E. Gray, D. Vicente, D. Planchard, L. Paz-Ares, et al.
Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer
J. Clin. Oncol., 40 (2022), pp. 1301-1311
[3]
C. Zhou, D. Huang, Y. Fan, X. Yu, Y. Liu, Y. Shu, et al.
Tislelizumab Versus Docetaxel in Patients With Previously Treated Advanced NSCLC (RATIONALE-303): A Phase 3, Open-Label, Randomized Controlled Trial
[4]
E.D. Kwon, C.G. Drake, H.I. Scher, K. Fizazi, A. Bossi, A.J. van den Eertwegh, et al.
Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial
[5]
L. Chen, J. Douglass, L. Kleinberg, X. Ye, A.E. Marciscano, P.M. Forde, et al.
Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma
[6]
N. Girard, J. Bar, P. Garrido, M.C. Garassino, F. McDonald, F. Mornex, et al.
Treatment Characteristics and Real-World Progression-Free Survival in Patients With Unresectable Stage III NSCLC Who Received Durvalumab After Chemoradiotherapy: Findings From the PACIFIC-R Study
J. Thorac. Oncol., 18 (2023), pp. 181-193
[7]
V. Amoroso, F. Gallo, A. Alberti, D. Paloschi, W. Ferrari Bravo, A. Esposito, et al.
Immune-related adverse events as potential surrogates of immune checkpoint inhibitors' efficacy: a systematic review and meta-analysis of randomized studies
ESMO Open, 8 (2023), Article 100787
[8]
A. Chennamadhavuni, L. Abushahin, N. Jin, C.J. Presley, A. Manne
Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors
Front. Immunol., 13 (2022), Article 779691
[9]
S. Das, D.B. Johnson
Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors
J. Immunother. Cancer, 7 (2019), p. 306
[10]
K. Suresh, K.R. Voong, B. Shankar, P.M. Forde, D.S. Ettinger, K.A. Marrone, et al.
Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors
J. Thorac. Oncol., 13 (2018), pp. 1930-1939
[11]
J. Gu, L. Shi, X. Jiang, J. Wen, X. Zheng, H. Cai, et al.
Severe immune-related adverse events of immune checkpoint inhibitors for advanced non-small cell lung cancer: a network meta-analysis of randomized clinical trials
Cancer Immunol. Immunother., 71 (2022), pp. 2239-2254
[12]
A. Berti, R. Bortolotti, M. Dipasquale, S. Kinspergher, L. Prokop, G. Grandi, et al.
Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer
Crit. Rev. Oncol. Hematol., 162 (2021), Article 103351
[13]
M.C. Korpics, R.R. Katipally, J. Partouche, D. Cutright, K.B. Pointer, C.M. Bestvina, et al.
Predictors of Pneumonitis in Combined Thoracic Stereotactic Body Radiation Therapy and Immunotherapy
Int. J. Radiat. Oncol. Biol. Phys., 114 (2022), pp. 645-654
[14]
S.G. Kroeze, C. Fritz, M. Hoyer, S.S. Lo, U. Ricardi, A. Sahgal, et al.
Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: A systematic review
Cancer Treat. Rev., 53 (2017), pp. 25-37
[15]
L.B. Marks, S.M. Bentzen, J.O. Deasy, F.M. Kong, J.D. Bradley, I.S. Vogelius, et al.
Radiation dose-volume effects in the lung
Int. J. Radiat. Oncol. Biol. Phys., 76 (2010), pp. S70-S76
[16]
K. Tsujino, T. Hashimoto, T. Shimada, E. Yoden, O. Fujii, Y. Ota, et al.
Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer
J. Thorac. Oncol., 9 (2014), pp. 983-990
[17]
S. Wang, Z. Liao, X. Wei, H.H. Liu, S.L. Tucker, C.S. Hu, et al.
Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT)
Int. J. Radiat. Oncol. Biol. Phys., 66 (2006), pp. 1399-1407
[18]
Y. Zhou, T. Yan, X. Zhou, P. Cao, C. Luo, L. Zhou, et al.
Acute severe radiation pneumonitis among non-small cell lung cancer (NSCLC) patients with moderate pulmonary dysfunction receiving definitive concurrent chemoradiotherapy: Impact of pre-treatment pulmonary function parameters
Strahlenther. Onkol., 196 (2020), pp. 505-514
[19]
S.L. Kwa, J.V. Lebesque, J.C. Theuws, L.B. Marks, M.T. Munley, G. Bentel, et al.
Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients
Int. J. Radiat. Oncol. Biol. Phys., 42 (1998), pp. 1-9
[20]
J. Bi, J. Qian, D. Yang, L. Sun, S. Lin, Y. Li, et al.
Dosimetric Risk Factors for Acute Radiation Pneumonitis in Patients With Prior Receipt of Immune Checkpoint Inhibitors
Front. Immunol., 12 (2021), Article 828858
[21]
S.M. Bentzen, L.S. Constine, J.O. Deasy, A. Eisbruch, A. Jackson, L.B. Marks, et al.
Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues
Int. J. Radiat. Oncol. Biol. Phys., 76 (2010), pp. S3-S9
[22]
N. Kita, N. Tomita, T. Takaoka, D. Okazaki, M. Niwa, A. Torii, et al.
Clinical and dosimetric factors for symptomatic radiation pneumonitis after stereotactic body radiotherapy for early-stage non-small cell lung cancer
Clin Transl Radiat Oncol, 41 (2023), Article 100648
[23]
S.Y. Song, W. Choi, S.S. Shin, S.W. Lee, S.D. Ahn, J.H. Kim, et al.
Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus
Lung Cancer, 66 (2009), pp. 89-93
[24]
L. Zou, L. Chu, F. Xia, L. Zhou, X. Yang, J. Ni, et al.
Is clinical target volume necessary?-a failure pattern analysis in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy using intensity-modulated radiotherapy technique
Transl Lung Cancer Res, 9 (2020), pp. 1986-1995
[25]
T. Cui, A. Zhang, J. Cui, L. Chen, G. Chen, H. Dai, et al.
Feasibility of omitting the clinical target volume under PET-CT guidance in unresectable stage III non-small-cell lung cancer: A phase II clinical trial
Radiother. Oncol., 181 (2023), Article 109505
[26]
K. Hsieh, D.R. Dickstein, J. Runnels, E.J. Lehrer, K. Rosenzweig, F.R. Hirsch, et al.
Radiotherapy and Immunotherapy in Lung Cancer
Biomedicines (2023), p. 11
[27]
S. Mi, N. Liang, Y. Zhang, Y. Zhang, F. Wang, L. Qiao, et al.
Effect of Sequence of Radiotherapy Combined With Immunotherapy on the Incidence of Pneumonitis in Patients With Lung Cancer: A Systematic Review and Network Meta-Analysis
Clin. Lung Cancer (2023)
[28]
M. Khunger, S. Rakshit, V. Pasupuleti, A.V. Hernandez, P. Mazzone, J. Stevenson, et al.
Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials
Chest, 152 (2017), pp. 271-281
[29]
F. Teng, M. Li, J. Yu
Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications
BMC Med., 18 (2020), p. 275
[30]
F. Cousin, C. Desir, S. Ben Mustapha, C. Mievis, P. Coucke, R. Hustinx
Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer
Radiother. Oncol., 157 (2021), pp. 47-55
点击查看原文
排版编辑:肿瘤资讯-Hanna











 苏公网安备32059002004080号
苏公网安备32059002004080号


