Highlights
•It is critical to develop a symptomatic radiation pneumonitis(RP) prediction model to enhance the safety of radiotherapy regimens in lung cancer patients with prior checkpoint inhibitors (ICIs) receipt followed by thoracic radiotherapy (TRT).
•We propose a novel combined model based on handcrafted (HCR) and deep learning-based (DLR) radiomics features obtained from three ROIs in planning CT images and clinical characteristics for predicting symptomatic RP in lung cancer patients receiving combined-mode treatment.
•The Fusion HCR model combing HCR features from three ROIs, outperformed models using a single ROI. The addition of DLR features improved the predictive performance of Fusion HCR model, with ResNet50 proving to be the most effective convolution layer.
•The model incorporating Fusion HCR features, DLR features based on ResNet50, and clinical characteristics had the highest predictive accuracy for symptomatic RP, achieving an AUC of 0.936 in the training cohort and 0.946 in the testing cohort.
Abstract
Purpose
This study aimed to combine clinical/dosimetric factors and handcrafted/deep learning radiomic features to establish a predictive model for symptomatic (grade ≥ 2) radiation pneumonitis (RP) in lung cancer patients who received immunotherapy followed by radiotherapy.
Materials and Methods
This study retrospectively collected data of 73 lung cancer patients with prior receipt of ICIs who underwent thoracic radiotherapy (TRT). Of these 73 patients, 41 (56.2 %) developed symptomatic grade ≥ 2 RP. RP was defined per multidisciplinary clinician consensus using CTCAE v5.0. Regions of interest (ROIs) (from radiotherapy planning CT images) utilized herein were gross tumor volume (GTV), planning tumor volume (PTV), and PTV-GTV. Clinical/dosimetric (mean lung dose and V5-V30) parameters were collected, and 107 handcrafted radiomic (HCR) features were extracted from each ROI. Deep learning-based radiomic (DLR) features were also extracted based on pre-trained 3D residual network models. HCR models, Fusion HCR model, Fusion HCR + ResNet models, and Fusion HCR + ResNet + Clinical models were built and compared using the receiver operating characteristic (ROC) curve with measurement of the area under the curve (AUC). Five-fold cross-validation was performed to avoid model overfitting.
Results
HCR models across various ROIs and the Fusion HCR model showed good predictive ability with AUCs from 0.740 to 0.808 and 0.740–0.802 in the training and testing cohorts, respectively. The addition of DLR features improved the effectiveness of HCR models (AUCs from 0.826 to 0.898 and 0.821–0.898 in both respective cohorts). The best performing prediction model (HCR + ResNet + Clinical) combined HCR & DLR features with 7 clinical/dosimetric characteristics and achieved an average AUC of 0.936 and 0.946 in both respective cohorts.
Conclusions
In patients undergoing combined immunotherapy/RT for lung cancer, integrating clinical/dosimetric factors and handcrafted/deep learning radiomic features can offer a high predictive capacity for RP, and merits further prospective validation.
Keywords
Immune checkpoint inhibitors;Radiation therapy;Radiomics;Deep learning;Radiation pneumonitis
Introduction
The use of immune checkpoint inhibitors (ICIs) in combination with thoracic radiotherapy (TRT) has gained significant traction in treating advanced cancers [1]. However, both ICIs and TRT are independent causative factors for pneumonitis, which can lead to unexpectedly high incidences of radiation pneumonitis (RP) with combinatorial therapy [2], [3]. To this extent, our previous study (of patients receiving ICIs prior to TRT) revealed a 40 % rate of symptomatic grade ≥ 2 RP, including two fatalities [4]. Therefore, it is critical to develop an RP prediction model (particularly for symptomatic RP) in the immunotherapy era.
Well before the introduction of immunotherapy for oncologic management, several studies determined that several dosimetric factors predicted for symptomatic RP, such as mean lung dose (MLD), volume of lungs receiving ≥ 20 Gy (V20), and volume of lungs receiving ≥ 30 Gy (V30) [5], [6], [7], [8]. However, dosimetry-associated RP prediction models exhibit an area under the curve (AUC) of only 0.66 [9], indicating a relatively low predictive capacity.
More recently, radiomics, which extracts high-level quantitative features from medical images, has emerged as a promising alternative [10], with prior studies highlighting the feasibility of using radiomic features from three regions of interest (ROIs), including planning target volume minus gross tumor volume (PTV-GTV) and total lung minus planning target volume (TL-PTV), derived from lung computed tomography (CT) images to predict symptomatic RP, exhibiting a promising area under the curve (AUC) of 0.82 and 0.80, respectively [11].
Moreover, combining dosimetric parameters and CT radiomic features improved the RP prediction model’s efficiency (AUC 0.84) [12]. Integrating clinical features such as tumor location, physical state, and smoking status can further enhance the RP prediction model’s performance [13], [14]. Finally, incorporating dosimetric parameters, CT radiomic features, age, and tumor (T) stage could further improve the prediction performance level (AUC = 0.94) under non-ICI context settings [11].
Given the unique intricacies of ICI therapy, however, the diagnostic accuracy of prior models for symptomatic RP prediction remain questionable. Therefore, to address this knowledge gap, we propose a novel combined model based on handcrafted (HCR) and deep learning-based (DLR) radiomic features obtained from three ROIs in planning CT images and clinical characteristics for predicting symptomatic RP in lung cancer patients with receipt of ICIs followed by TRT.
Methods and materials
Patients and treatment
This retrospective study received approval from the Ethics Committees of Hubei Cancer Hospital (A) and First Affiliated Hospital of Yangtze University (B). It entailed a review of lung cancer patients who underwent TRT at these two institutions between March 2020 and July 2021. Inclusion criteria were as follows: (1) newly-diagnosed and pathologically confirmed lung cancer; (2) receipt of ICIs followed by TRT; (3) availability of clinicopathological and dosimetric data. Exclusion criteria were: (1) absence of imaging when RP was diagnosed; (2) lack of follow-up; (3) previous history of TRT. Ultimately, the dataset consisted of 59 patients from Institute A and 14 patients from Institute B. All eligible patients were shuffled and divided into a training cohort (n = 59) and a testing cohort (n = 14) at an 8:2 ratio.
The diagnosis of RP was established by a multidisciplinary oncologic team consisting of at least one radiation oncologist, one radiologist, and one pulmonologist. The diagnosis was based on the presence of diffuse lung abnormalities limited to (or largely limited to) the radiation field of the lung on CT scans, as well as clinical symptoms, while excluding other possible causes such as infection or checkpoint inhibitor-related pneumonitis (CIP) [15]. At both institutions, all patients received a chest CT examination prior to RT, which was repeated one month and three months after RT to assess efficacy and potential RP. Symptomatic RP was defined as grade ≥ 2 RP using the Common Terminology Criteria for Adverse Events, version 5. All suspected diagnoses of pneumonitis were reviewed by a multidisciplinary committee comprising at least one radiologist, pulmonologist, and oncologist, who analyzed follow-up images as well as clinical records.
Clinical imaging and radiotherapy planning
At both institutions, radiotherapy planning CT (pCT) images were acquired on a Philips 16-slice Brilliance Big Bore CT scanner with a tube voltage of 120 kVp, tube current ranging from 100 to 200 mA, and a rotation time of 0.5–0.8 s. The images were acquired under free-breathing conditions and had a slice thickness and slice interval of 5 mm. To ensure consistent radiographic quality among CT images, a spline interpolation algorithm was used to resample all images to a uniform image spacing of 1 mm × 1 mm × 1 mm.
In this study, all patients from Institute A received thoracic RT with intensity modulated radiation therapy (IMRT) using the Varian Edge linear accelerator (6 MV, 1400 MU/min), and all patients from Institute B received thoracic IMRT using Varian Vital Beam (6 MV, 600 MU/min). For the analysis, three ROIs were selected from the pCT images: the GTV, PTV, and PTV-GTV (Fig. 1). GTV was defined as the visible primary tumor and metastatic lymphadenopathy, while PTV was defined as a 5 mm uniform expansion of the GTV [16]. PTV-GTV was obtained by excluding the GTV from PTV.
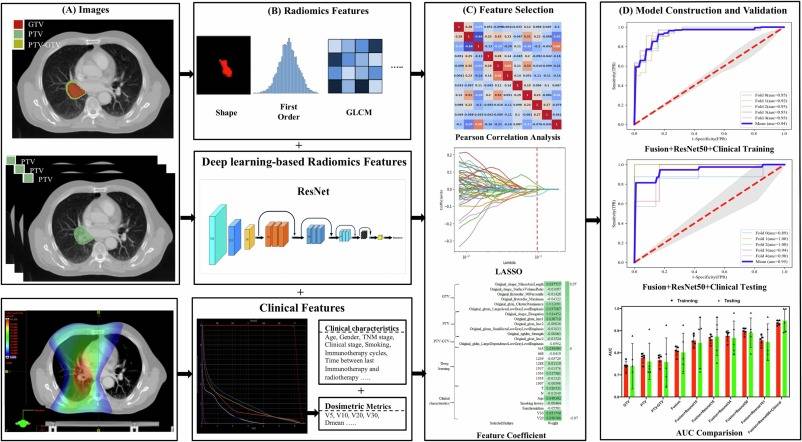
Fig. 1. This flow chart summarizes the steps performed in this study. The sequences and details of these steps are as follows: (A) Labeling of the regions of interest (ROIs): 3 ROIs (GTV, PTV, and PTV-GTV) were selected from the radiotherapy planning CT (pCT) images. (B) Feature extraction: this step involved extracting dosimetric metrics, demographic- and treatment-related data, handcrafted radiomic features (HCR) from each ROI, and deep learning-based radiomic (DLR) features from PTV coverage layers using pre-trained 3D residual network (ResNet) models. (C) Feature selection: normalization was initially performed. To avoid overfitting, Pearson correlation coefficients were calculated between each pair of features, and redundant features with a correlation coefficient greater than 0.9 were removed. The least absolute shrinkage and selection operator (LASSO) regression technique was used to reduce the dimensionality of the dataset and prevent overfitting. (D) Model construction and validation: logistic Regression models were constructed using the sklearn library. The validity of the prediction models was evaluated using five-fold cross-validation on the dataset.
For each patient, in addition to obtaining clinical (demographic- and treatment-related) data, dosimetric metrics were also collected, including the MLD, V5, V10, V20, and V30 within TL-GTV, along with the volume of GTV, PTV, and total lungs.
Handcrafted radiomic features
A total of 107 radiomic features were extracted from each ROI, which included 14 shape features, 18 first order features, 24 Gray Level Co-occurrence Matrix (GLCM) features, 16 Gray Level Run-Length Matrix (GLRLM) features, 16 Gray Level Size Zone Matrix (GLSZM) features, 5 Neighborhood Gray-Tone Difference Matrix (NGTDM) features, and 14 Gray Level Dependence Matrix (GLDM) features. Handcrafted radiomic feature extraction was performed using PyRadiomics software (https://pyradiomics.readthedocs.io/) [17].
Deep learning-based radiomic features
This investigation also employed deep learning-based radiomic (DLR) features that were extracted from PTV coverage layers. Pretrained 3D residual network (ResNet) models were utilized for feature extraction. ResNet is a subgroup of convolutional neural network (CNN) methods that are renowned for their effective management of both spatial and temporal features simultaneously [18]. Transfer learning was used for feature extraction, wherein a Kinetics-400 (https://arxiv.org/abs/1705.06950) pre-trained 3D-ResNet was employed [19]. To begin, the focal layer was eliminated through the following methodology: (1) conducting element-wise multiplication of the image and the mask, (2) setting the non-ROI region of the image to 0, (3) eliminating all the sections containing 0, and exporting the sections without 0. Subsequently, the image containing the ROI layer was fed into the ResNet model and deep features were extracted from the penultimate layer of each ResNet model. Finally, ResNet10 ∼ 34 yielded 513 DLR features, while ResNet50 ∼ 101 generated 2048 DLR features. The specifics of ROI segmentation and the flowchart for radiomics and ResNet are depicted in Fig. 1. The following link can be accessed for additional code details: https://github.com/kenshohara/3D-ResNets-PyTorch.
Feature selection and model construction
Prior to feature selection, normalization using z-scores with a mean of zero and standard deviation of one was performed. To avoid overfitting, we calculated Pearson correlation coefficients between each pair of features, removing one of two redundant features with r > 0.9 when present. Furthermore, we utilized least absolute shrinkage and selection operator (LASSO) regression to reduce the dataset’s feature count and prevent overfitting. The most relevant features were selected through five-fold cross-validation. All models were constructed using the Logistic Regression algorithm from the sklearn library. To evaluate the prediction models’ validity, five-fold cross-validation was also performed on the dataset [20]. The outcomes included the training AUC, testing AUC, accuracy, precision, recall, and F1-score.
This study established four different symptomatic RP prediction models, including HCR models, Fusion HCR model, Fusion HCR + ResNet models, and Fusion HCR + ResNet + Clinical models. The handcrafted features across various ROIs were used to construct the HCR models, and the fusion of handcrafted features from three ROIs was employed to create the Fusion HCR model. The Fusion HCR + ResNet models utilized a combination of fusion handcrafted features and ResNet-based DLR features. For the development of the Fusion HCR + ResNet + Clinical models, clinical characteristics (including dosimetric parameters) were included in conjunction with HCR and DLR features.
Statistical analysis
All data analyses were conducted using Python software (version 4.1.0). Based on the linear-quadratic model, MiM software was used to convert the physical dose distribution into EQD2, assuming α/β of 10 for the target and 3 for the normal lung [21]. Differences between variables were evaluated by the Pearson Chi-square test or Student’s t-test, and p < 0.05 was considered statistically significant. The symptomatic RP prediction models were constructed through the application of the Logistic Regression algorithm. In order to assess the discriminative capacity of the models, the receiver operating characteristic (ROC) curve was utilized, with the performance being evaluated through the calculation of the AUC, accuracy, recall, precision, and F1 Score.
Results
Table 1 illustrates the TRT-related characteristics of the population. Specifically, 48 patients received conventional RT, 20 underwent hypofractionated RT, and 5 received stereotactic body radiation therapy (SBRT). The median EQD2 dose across all patients was 60 Gy (interquartile range [IQR], 56–64 Gy). To account for the heterogeneity in dose/fractionation schemes, all plans were standardized using EQD2 doses, resulting in a median (IQR) MLD, V5, V10, V20, and V30 of 11.0 Gy (7.9–13.8), 35.9 % (26.1–48.5), 25.8 % (19.0–35.6), 20.4 % (12.9–24.2), and 14.6 % (8.9–18.7), respectively.
Table 1. Thoracic Radiotherapy Characteristics.
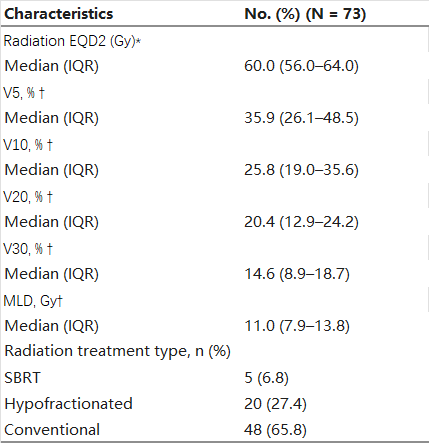
Abbreviations: EQD2, equivalent dose in 2 Gy fractions; IQR: interquartile range; Vx: volume of lung receiving ≥ xGy; MLD: mean lung dose; ICIs: immune checkpoint inhibitors; SBRT: stereotactic body radiation therapy.
*
Assuming an α/β of 10.
†
Following conversion of all dose-fractionation schemes to EQD2 based on the LQ model, assuming an α/β of 3.
Table 2 displays demographic characteristics of the study cohort. Among the 73 patients included in the analysis, a total of 41 individuals (56.2 %) exhibited grade ≥ 2 RP. Notably, no significant differences were observed between the non-symptomatic and symptomatic RP groups with respect to any clinical or dosimetric parameter (p > 0.05 for all), with exception of V20 (p = 0.046) and V30 (p = 0.031).
Table 2. Patient characteristic.
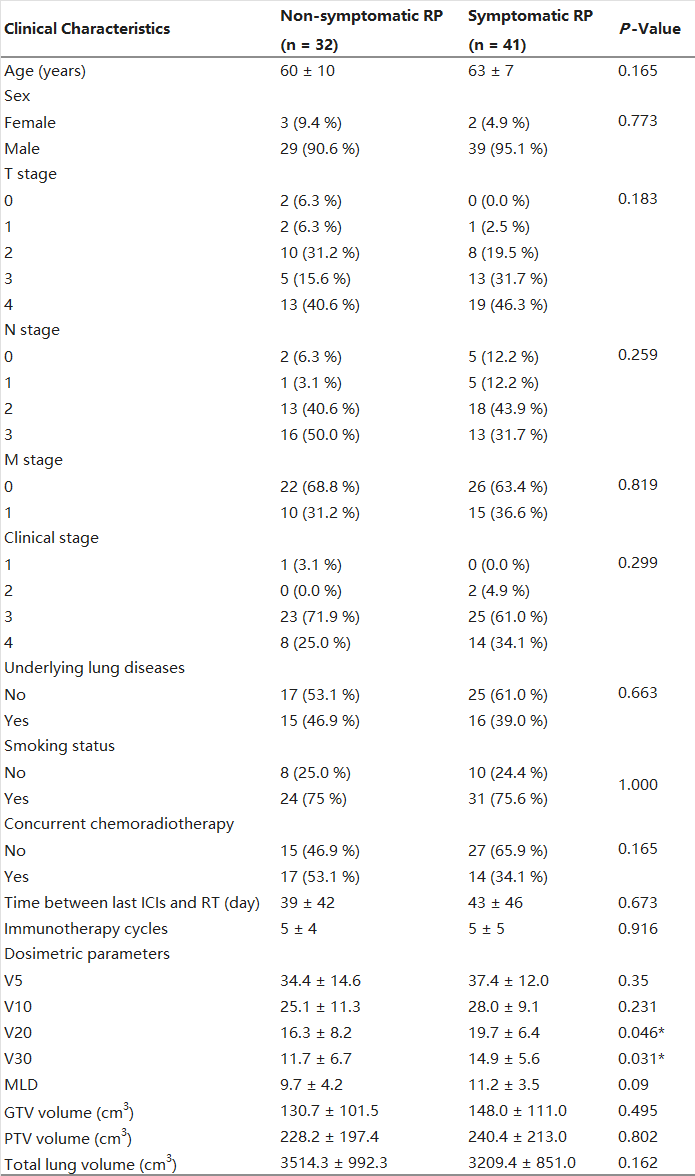
Abbreviations: GTV, gross tumor volume; ICIs, immune checkpoint inhibitors; PTV, planning tumor volume; RP, radiation pneumonitis; RT, Radiotherapy.
The HCR models constructed using HCR features obtained from PTV, PTV-GTV, and TL-GTV displayed favorable performance in prognosticating symptomatic RP, with mean AUC values ranging between 0.740 and 0.777 in the training cohort and 0.739–0.761 in the testing cohort (Table 3 and Fig. 2). Furthermore, integration of HCR features extracted from three ROIs led to an improved overall performance of the Fusion HCR model, achieving mean AUC values of 0.808 and 0.802 in the training and testing cohorts, respectively.
Table 3. The Performance of the HCR Models and Fusion HCR Model.
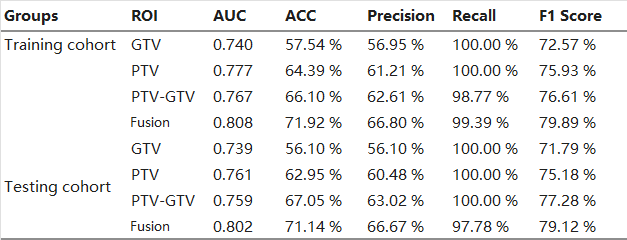
Abbreviations: ACC, accuracy; AUC, area under the curve; GTV, gross tumor volume; HCR, handcrafted radiomics; PTV, planning target volume; ROI, region of interest.
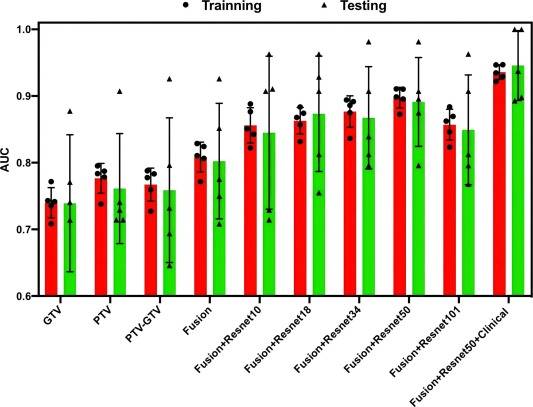
Fig. 2. The Box-and-Whisker plots of AUC values for all the models.
Overall, the addition of DLR features may improve model effectiveness. Specifically, with the increase of the convolutional layer, the efficiency of the model gradually increased from average AUC values ranging from 0.782 to 0.856 in the training cohort, and 0.781–0.845 in the testing cohort for the Fusion HCR + ResNet10 Models, reaching a peak at Fusion HCR + ResNet50models, with an average AUC value ranging from 0.826 to 0.898 in the training cohort and 0.821–0.898 in the testing cohort, and ending with an average AUC value ranging from 0.786 to 0.857 in the training cohort and 0.779–0.849 in the testing cohort for Fusion HCR + ResNet101 Models. Of these models, the Fusion HCR + ResNet50 model demonstrated highly competitive performance, with AUCs of 0.897 and 0.897 in the training and testing cohorts, respectively. Detailed results can be observed in both Table 4 and Fig. 2.
Table 4. The performance of the Fusion + ResNet Models.
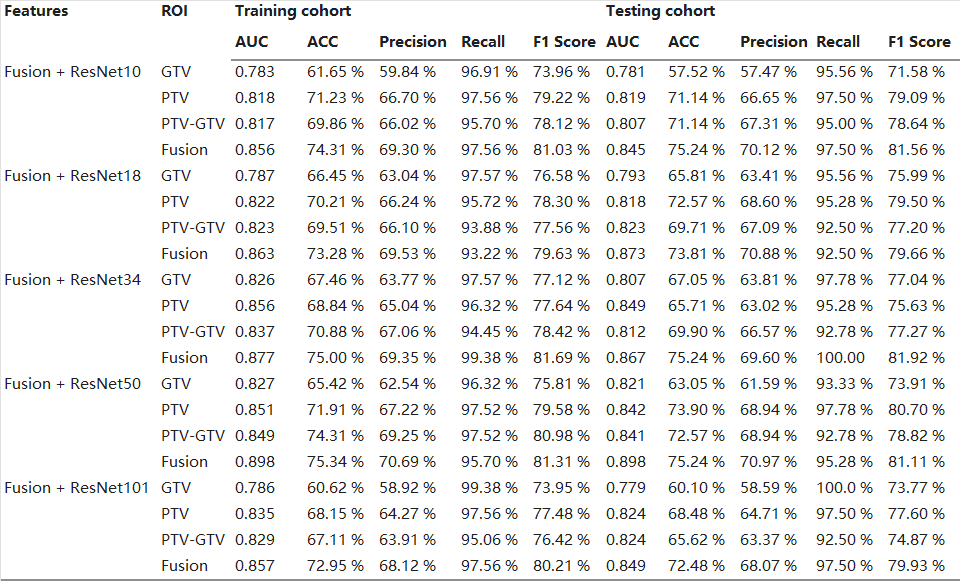
Abbreviations: ACC, accuracy; AUC, area under the curve; GTV, gross tumor volume; PTV, planning target volume; ROI, region of interest.
Accounting for clinical characteristics, we constructed the optimal predictive model (Fusion HCR + ResNet50 + Clinical Model) for symptomatic RP, which was able to achieve an AUC of 0.936 in the training cohort and 0.946 in the testing cohort (Table 5 and Fig. 2). Fig. 3 provides a comprehensive list of the radiomic features and clinical characteristics incorporated in the model, encompassing 13 HCR features, 8 DLR features, as well as 7 clinical/dosimetric characteristics (T stage, N stage, age, smoking status, concurrent chemoradiotherapy, V20, and V30). Moreover, Fig. 4 depicts the ROC curves and the five-fold cross-validation confusion matrix of the Fusion HCR + ResNet50 + Clinical model in the testing cohort.
Table 5. The Performance of Five-Fold Validation in Fusion + Resnet 50 + Clinical Model.
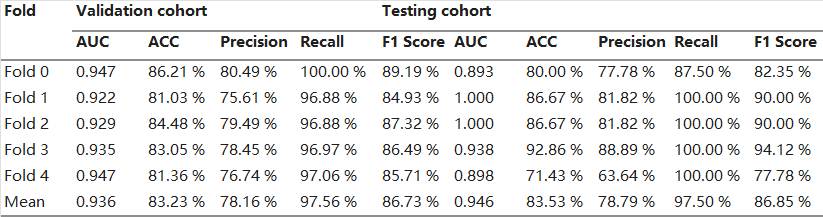
Abbreviations: ACC, accuracy; AUC, area under the curve; GTV, gross tumor volume; PTV, planning target volume; ROI, region of interest.
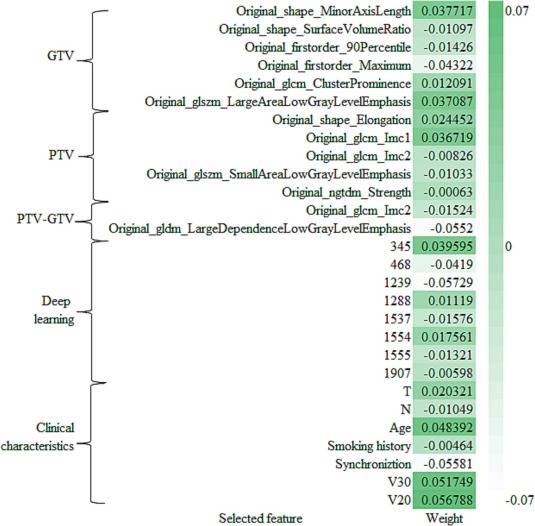
Fig. 3. Details of the HCR, DLR and clinical features included in the Fusion HCR + ResNet50 + Clinical model. HCR, handcrafted radiomics; DLR, deep learning-based radiomics.
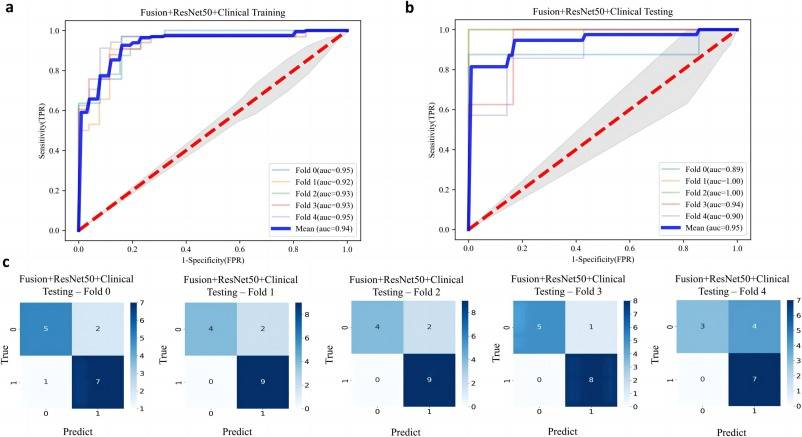
Fig. 4. The ROC curves in the training cohort (a) and testing cohort (b) for the Fusion HCR + ResNet50 + Clinical model. AUC values are expressed as mean standard deviation of the five training and validation sets from the 5-fold cross-validation. (c) The confusion matrices with heatmaps of the five validation sets of the ResNet models. The numbers in each colored box represent the percentage of instances between the true and the predicted classes obtained by the ResNet models. ROC, receiver-operating characteristic; AUC, area under the curve. ResNet, residual network.
Discussion
To date, publications attempting to predict radiation pneumonitis (RP) have not been in context of immunotherapy; as such, predicting RP in the setting of combined ICI-TRT therapy remains unknown, which was the primary impetus for this investigation. After constructing four different prediction models, we found that the Fusion HCR model, which combines HCR features from three ROIs, outperformed models using a single ROI. Furthermore, adding DLR features to the Fusion HCR model improved its performance, with ResNet50 proving to be the most effective convolution layer. Ultimately, the model incorporating Fusion HCR features, DLR features based on ResNet50, and clinical characteristics had the highest predictive accuracy for symptomatic RP, achieving an AUC of 0.936 in the training cohort and 0.946 in the testing cohort.
The traditional HCR model has been widely studied as an imaging biomarker in oncology to predict prognosis and treatment response [22], [23]. Previous investigations have demonstrated the potential of radiomic features extracted from pre-treatment planning CT images [24], [25] and [18F]-2-fluoro-2-deoxyglucose positron emission tomography/computed tomography (FDG PET/CT) images [26], [27] and the combination thereof [28] as markers for symptomatic RP prediction. Additionally, multi-region radiomic features have improved predictive performance compared to the whole lung ROI (AUC = 0.68) [29] with AUC values of 0.78 and 0.84 [30], [31], respectively. Our results showed that the fusion of multi-region HCR features can improve the model output, given that the AUC value increased from 0.740 to 0.808. Although this is generally consistent with previous studies, the primary difference was that previous studies reported differential efficacy based on various ROIs [30], [31], but the present work reported similar and satisfactory predictive ability in models based on various ROIs. These results indicated that the high dose regions represented by these ROIs were both closely related to the development of symptomatic RP, which could be considered for RT treatment planning. Furthermore, traditional handcrafted radiomics face challenges with regards to reproducibility and interpretability [32], [33], creating opportunities for deep learning-based methods such as Resnet in CNN [34] that capture textural information in initial convolutional layers, and can be visualized using advanced techniques. Previous RP radiomic-based prediction methods have employed only a single traditional handcrafted or deep learning method (or have compared two methods). The present study employed a combination of handcrafted features and deep features, as it offers the potential to bridge the gap between traditional radiomics and deep learning techniques, while also enhancing interpretability [35], [36]. The results of this study demonstrated that the inclusion of deep features through Resnet improved the performance of HDR models, resulting in an AUC increase from 0.808 to 0.898. This finding is consistent with previous studies that examined idiopathic pulmonary fibrosis (IPF) and non-IPF interstitial lung diseases (ILDs) [37] and the prediction of axillary lymph node metastasis of breast cancer [38] when combining handcrafted radiomics features with deep learning features. Compared with ResNet18 and 34, ResNet50′s higher performance may be mainly attributed to its deeper convolution layers and plentiful feature maps, which reduce the risk of underfitting as well as assist in extracting more information from the data and the subsequent learning thereof. Theoretically, ResNet101 is expected to outperform ResNet50; however, our study exhibited superior performance with the latter. This could be attributed to ResNet101′s pronounced susceptibility to overfitting with simple datasets or limited samples, likely due to its higher computational cost. Additionally, the selective attention mechanism of ResNet101 may be prone to overlearning in the absence of sufficient training, leading to decreased performance. Hence, researchers should carefully evaluate the suitability of models based on the specific problem and dataset they are studying [39], [40]. Recently, a similar study [41] focusing on the integration of dosimetric parameters and machine learning-based radiomic methods was employed to predict RP. However, it should be noted that the current study differs from the aforementioned research in several key aspects. First, the predicted outcome in the cited study was any-grade RP rather than symptomatic RP. However, symptomatic RP is an important indicator that impacts the subsequent management measures for patients. Furthermore, the dataset utilized in the aforementioned study mainly comprised radiotherapy patients, with a limited number (n = 11) of ICI patients included.
Previous RP prediction models have demonstrated that the inclusion of clinical characteristics, including dosimetric parameters, results in improved discrimination ability (AUC ranged from 0.782 to 0.940) compared to models that exclude clinical characteristics (AUC ranged from 0.690 to 0.871). Similarly, the integration of clinical features in the current study increased the AUC from 0.898 (Fusion HCR + ResNet 50 model) to 0.936 (Fusion HCR + ResNet 50 + Clinical model). Seven clinically accessible characteristics were ultimately incorporated into the Fusion HCR + ResNet 50 + Clinical model, which may have potential for pre-treatment assessment of symptomatic RP in lung cancer. Jiang et al. also incorporated T stage into the RP radiomics model, achieving favorable predictive performance [11]. Notably, our study identified tumor T and N stage as predictors, potentially due to their impact on the definition of other risk factors, including GTV, PTV, and lung volume [42], [43], [44], [45]. Based on the clinical features evaluated herein, age [6], [46], [47], [48] and smoking status [49], [50] were found to be frequent predictors of symptomatic RP, as they may reflect the overall physical condition, lung function, and any underlying lung disease. Furthermore, previous traditional RP prediction methods have emphasized the importance of dosimetric parameters; among various dosimetric parameters, V20 and MLD have consistently been associated with symptomatic RP [4], [6], [30], [51], [52], and our previous investigation confirmed the predictive capacity of V20 in combined ICI/TRT patients [4]. However, the AUC value for symptomatic RP prediction using V20 was only 0.703, and prediction models were not established in the previous study. Comparatively, herein, significant differences in V20 and V30 were simultaneously observed between the symptomatic and non-symptomatic RP groups, and the comprehensive prediction model (which included both V20 and V30) exhibited an AUC of 0.936 in the training cohort and 0.946 in the testing cohort. In addition, a series of radiomic features were also obtained in this study, such as shape-based features, which may be related to the extent of tumor invasion. These features could influence the delineation of target areas during planning, leading to sharper tumor margins (which tend to be more prone to RP) [53]. Moreover, texture features including GLCM-based features were also extracted, representing the heterogeneity of the tumor. Tumors with higher heterogeneity and more irregular edges tend to be more susceptible to RP [53]. Furthermore, the convolutional form of DLR features aids in identifying deep image texture distribution information. Previous data have shown that the information of the whole lung (outside of the tumor itself) also has a certain predictive effect on RP [11]. In the present study, the DLR features of whole lung data (encompassing the PTV as well) may serve as a complementary approach to the heterogeneity of tumors, thereby improving the accuracy of RP prediction.
The current investigation offers invaluable insights into the management of patients undergoing RT in conjunction with ICIs. For instance, our study revealed a substantial risk of developing RP in such individuals, thereby highlighting a number of interventions that could be considered. Future efforts should be directed at devising strategies aimed at minimizing the extent of lung tissue exposed to radiation, quantified by the PTV volume as well as the V20. Notably, one approach to reducing the radiation dose to healthy tissues involves the exclusion of the CTV [54], and in order to explore this issue we have launched multiple clinical trials (NCT06020430, NCT06037733). In addition to these ideas, prophylactic medications to prevent RP could also be considered. Of noteworthy mention is a study by Liu et al., which illustrated that the administration of thymus thymosin α1 effectively decreased the incidence of grade ≥ 2 RP [55]. To better address this issue, our group has developed an investigational single-arm, open-label, phase II trial (NCT05801133) examining the use of pirfenidone to manage RP in this particular population. Lastly, it is highly recommended to closely monitor these patients during the course of radiation therapy as well as during follow-up visits.
Additionally, it is noteworthy that five patients were treated with SBRT in this study. SBRT is considered the standard therapy for patients with inoperable tumors or those who refuse surgical resection in early stage non-small cell lung cancer (NSCLC). SBRT exhibits immune activation effects by modulating the local environment and activating local and systemic innate and adaptive immune mechanisms, thus further enhancing the effectiveness of immunotherapy. Furthermore, immunotherapy may also sensitize tumors to radiation by promoting the normalization of tumor blood vessels, improving tissue perfusion, and reducing intratumoral hypoxia [56]. Some studies have also illustrated the combination of SBRT and ICIs to have promising therapeutic effects [57], [58]. As a result, the combination of SBRT and ICIs is being increasingly utilized in clinical practice. However, it should be noted that both SBRT and ICI therapy independently carry the risk of pneumonitis. When compared to monotherapy, the risk of grade 3 pneumonitis appears to be higher with the combination of SBRT and ICIs [59]. It is possible that ICI therapy triggers an inflammatory reaction involving infiltrating lymphocytes and cytokines in previously irradiated areas, thus contributing to RP [60]. However, the exact mechanism requires further investigation. Prophylactic medication against pneumonitis should be considered, including the use of thymosin α1 [55] and pirfenidone [60].
This study has several limitations. First, although the determination of RP grade herein was based on multidisciplinary clinician consensus, retrospective diagnosis cannot eliminate uncertainties or other biases. As such, prospective validation is required. Second, all patients herein received ICIs followed by RT, so these data may not necessarily be applicable to other forms of ICI/RT sequencing. Third, this study employed a large set of radiomic features to build a prediction model for a limited dataset, which may increase the risk of overfitting. To mitigate this risk, we used Pearson correlation analysis and LASSO to remove redundant features before feature selection, and five-fold cross-validation to reduce overfitting in nonlinear regression when constructing prediction models. In addition, the dataset was obtained from two centers and a separate verification set was used to further reduce the risk of overfitting. Finally, the current study incorporated only limited clinical features to improve the model’s predictive accuracy. In future studies, other clinical features that have been previously associated with RP, such as lung function indicators, metabolic data, biological factors, and novel dosimetric parameters [61] could be included to assess whether the predictive accuracy can be further improved.
In conclusion, this study demonstrated that the integration of handcrafted radiomic features from multiple regions, together with deep learning-based radiomic features and relevant clinical characteristics, could enhance the predictive performance of symptomatic RP in lung cancer patients receiving ICIs followed by RT. These findings lay an important foundation for assessing the safety of combinatorial therapy. Moreover, more accurate evaluation of symptomatic RP in high-risk individuals can enable closer monitoring, earlier intervention, and potentially better clinical outcomes.
Ethics approval and consent to participate
This retrospective study received approval from the Ethics Committees of Hubei Cancer Hospital (A) and First Affiliated Hospital of Yangtze University(B).
Consent for publication
All authors have read and approved the manuscript and agree with submission to Biomarker Research.
Funding
Zilong Yuan was supported by National Natural Science Foundation of China supported this study (No. 62076257), and Applied Basic Research Programme of Wuhan (No. 2020020601012239). Guang Han was supported by Cancer Research Program of National Cancer Center of China (NCC201917B03) and Bethune Research Foundation of China (flzh202121). Yulin Liu was supported by National key Research and Development Program of China (2022YFC2410000), Hubei Provincial Key Technology Foundation of China (2021ACA013), National key Research and Development Project of China (2018YFA0704000). Jianping Bi was supported by Research Projects of Biomedical Center of Hubei Cancer Hospital (grant number 2022SWZX17).
Availability of data and materials
All relevant data and materials have been included in the article. Further inquiries can be directed to the corresponding authors.
CRediT authorship contribution statement
Tingting Nie: Writing – original draft. Zien Chen: Methodology, Formal analysis. Jun Cai: Data collection. Shuangquan Ai: Formal analysis. Xudong Xue: Data collection. Mengting Yuan: Data collection. Chao Li: Data collection. Liting Shi: Review & editing. Yulin Liu: Review & editing. Vivek Verma: Review & editing. Jianping Bi: Review & editing. Guang Han: Review & editing. Zilong Yuan: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
[1]
W.S.M.E. Theelen, D. Chen, V. Verma, B.P. Hobbs, H.M.U. Peulen, J.G.J.V. Aerts, et al.
Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials
Lancet Respir Med, 9 (2021), pp. 467-475
[2]
D.Y. Wang, J.E. Salem, J.V. Cohen, S. Chandra, C. Menzer, F. Ye, et al.
Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis
[3]
F. Cousin, C. Desir, S. Ben Mustapha, C. Mievis, P. Coucke, R. Hustinx
Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer
RadiotherOncol, 157 (2021), pp. 47-55
[4]
J. Bi, J. Qian, D. Yang, L. Sun, S. Lin, Y. Li, et al.
Dosimetric risk factors for acute radiation pneumonitis in patients with prior receipt of immune checkpoint inhibitors
Front Immunol, 13 (2022), Article 828858
[5]
W.Y. Pan, C. Bian, G.L. Zou, C.Y. Zhang, P. Hai, R. Zhao, et al.
Combing NLR, V20 and mean lung dose to predict radiation induced lung injury in patients with lung cancer treated with intensity modulated radiation therapy and chemotherapy
Oncotarget, 8 (2017), pp. 81387-81393
[6]
J.M. Ryckman, M. Baine, J. Carmicheal, F. Osayande, R. Sleightholm, K. Samson, et al.
Correlation of dosimetric factors with the development of symptomatic radiation pneumonitis in stereotactic body radiotherapy
Radiat Oncol, 15 (2020), p. 33
[7]
M. Nakamura, H. Nishimura, M. Nakayama, H. Mayahara, H. Uezono, A. Harada, et al.
Dosimetric factors predicting radiation pneumonitis after CyberKnife stereotactic body radiotherapy for peripheral lung cancer
Br J Radiol, 89 (2016), Article 20160560
[8]
B. Liang, H. Yan, Y. Tian, X. Chen, L. Yan, T. Zhang, et al.
Dosiomics: extracting 3D spatial features from dose distribution to predict incidence of radiation pneumonitis
Front Oncol, 9 (2019), p. 269
[9]
Y. Luo, D.L. McShan, M.M. Matuszak, D. Ray, T.S. Lawrence, S. Jolly, et al.
A multiobjective Bayesian networks approach for joint prediction of tumor local control and radiation pneumonitis in nonsmall-cell lung cancer (NSCLC) for response-adapted radiotherapy
Med Phys, 4 (2018), p. 10
[10]
P. Lambin, R.T.H. Leijenaar, T.M. Deist, J. Peerlings, E.E.C. de Jong, J. van Timmeren, et al.
Radiomics: the bridge between medical imaging and personalized medicine
Nat Rev Clin Oncol, 14 (2017), pp. 749-762
[11]
W. Jiang, Y. Song, Z. Sun, J. Qiu, L. Shi
Dosimetric factors and radiomics features within different regions of interest in planning CT images for improving the prediction of radiation pneumonitis
Int J Radiat Oncol Biol Phys, 110 (2021), pp. 1161-1170
[12]
D. Kawahara, N. Imano, R. Nishioka, K. Ogawa, T. Kimura, T. Nakashima, et al.
Prediction of radiation pneumonitis after definitive radiotherapy for locally advanced non-small cell lung cancer using multi-region radiomics analysis
Sci Rep, 11 (2021), p. 16232
[13]
D.A. Palma, S. Senan, K. Tsujino, R.B. Barriger, R. Rengan, M. Moreno, et al.
Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis
Int J Radiat Oncol Biol Phys, 85 (2013), pp. 444-450
[14]
L. Ma, W. Ye, Q. Li, B. Wang, G. Luo, Z. Chen, et al.
Subjective Global Assessment (SGA) score could be a predictive factor for radiation pneumonitis in lung cancer patients with normal pulmonary function treated by intensity-modulated radiation therapy and concurrent chemotherapy
Clin Lung Cancer, 19 (2018), pp. e211-e217
[15]
J. Cheng, Y. Pan, W. Huang, K. Huang, Y. Cui, W. Hong, et al.
Differentiation between immune checkpoint inhibitor-related and radiation pneumonitis in lung cancer by CT radiomics and machine learning
Med Phys, 49 (2022), pp. 1547-1558
[16]
U. Nestle, D. De Ruysscher, U. Ricardi, X. Geets, J. Belderbos, C. Pöttgen, et al.
ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer
Radiother Oncol, 127 (2018), pp. 1-5
[17]
J.J.M. van Griethuysen, A. Fedorov, C. Parmar, A. Hosny, N. Aucoin, V. Narayan, et al.
Computational radiomics system to decode the radiographic phenotype
Cancer Res, 77 (2017), p. e104
[18]
X. Zheng, Z. Yao, Y. Huang, Y. Yu, Y. Wang, Y. Liu, et al.
Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer
Nat Commun, 11 (2020), p. 1236
[19]
K. He, X. Zhang, S. Ren, J. Sun
Spatial pyramid pooling in deep convolutional networks for visual recognition
IEEE Trans Pattern Anal Mach Intell, 37 (2015), pp. 1904-1916
[20]
T. van der Ploeg, E.W. Steyerberg
Feature selection and validated predictive performance in the domain of Legionella pneumophila: a comparative study
BMC Res, Notes 9 (2016), pp. 1-7
[21]
C. Schröder, I. Stiefel, S. Tanadini-Lang, I. Pytko, E. Vu, M. Guckenberger, et al.
Re-irradiation in the thorax - An analysis of efficacy and safety based on accumulated EQD2 doses
Radiother Oncol, 152 (2020 Nov), pp. 56-62
[22]
T.P. Coroller, P. Grossmann, Y. Hou, E. Rios Velazquez, R.T. Leijenaar, G. Hermann, et al.
CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma
Radiother Oncol, 114 (2015), pp. 345-350
[23]
H. Aerts, E.R. Velazquez, R. Leijenaar, C. Parmar, P. Lambin
Corrigendum: decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach
Nat Commun, 5 (2014), p. 4006
[24]
T.A. Hirose, H. Arimura, K. Ninomiya, T. Yoshitake, J.I. Fukunaga, Y. Shioyama
Radiomic prediction of radiation pneumonitis on pretreatment planning computed tomography images prior to lung cancer stereotactic body radiation therapy
Sci Rep, 10 (2020), p. 20424
[25]
A. Cunliffe, S.G. Armato 3rd, R. Castillo, N. Pham, T. Guerrero, H.A. Al-Hallaq
Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development
Int J Radiat Oncol Biol Phys, 91 (2015), pp. 1048-1056
[26]
R. Castillo, N. Pham, S. Ansari, D. Meshkov, S. Castillo, M. Li, et al.
Pre-radiotherapy FDG PET predicts radiation pneumonitis in lung cancer
Radiat Oncol, 13 (2014), p. 74
[27]
R. Castillo, N. Pham, E. Castillo, S. Aso-Gonzalez, S. Ansari, B. Hobbs, et al.
Pre-Radiation therapy Fluorine 18 Fluorodeoxyglucose PET helps identify patients with esophageal cancer at high risk for radiation pneumonitis
Radiology, 275 (2015), pp. 822-831
[28]
G.J. Anthony, A. Cunliffe, R. Castillo, N. Pham, T. Guerrero, S.G. Armato 3rd, et al.
Incorporation of pre-therapy 18 F-FDG uptake data with CT texture features into a radiomics model for radiation pneumonitis diagnosis
Med Phys, 44 (2017), pp. 3686-3694
[29]
S.P. Krafft, A. Rao, F. Stingo, T.M. Briere, L.E. Court, Z. Liao, et al.
The utility of quantitative CT radiomics features for improved prediction of radiation pneumonitis
Med Phys, 45 (2018), pp. 5317-5324
[30]
D. Kawahara, N. Imano, R. Nishioka, K. Ogawa, T. Kimura, T. Nakashima, et al.
Prediction of radiation pneumonitis after definitive radiotherapy for locally advanced non-small cell lung cancer using multi-region radiomics analysis
Sci Rep, 11 (2021), p. 16232
[31]
B. Li, G. Ren, W. Guo, J. Zhang, S.K. Lam, X. Zheng, et al.
Function-Wise Dual-Omics analysis for radiation pneumonitis prediction in lung cancer patients
Front Pharmacol, 19 (2022), Article 971849
[32]
Y. Xu, A. Hosny, R. Zeleznik, C. Parmar, T. Coroller, I. Franco, et al.
Deep learning predicts lung cancer treatment response from serial medical imaging
Clin Cancer Res, 25 (2019), pp. 3266-3275
[33]
A. Hosny, C. Parmar, T.P. Coroller, P. Grossmann, R. Zeleznik, A. Kumar, et al.
Deep learning for lung cancer prognostication: a retrospective multi-cohort radiomics study
PLoS Med, 15 (2018), p. e1002711
[34]
G. Huang, Z. Liu, L. Van Der Maaten, K.Q. Weinberger
Densely connected convolutional networks
Comput Vision Pattern Recognit, 1 (2017), pp. 4700-4708
[35]
A. Hosny, H.J. Aerts, R.H. Mak
Handcrafted versus deep learning radiomics for prediction of cancer therapy response
Lancet Digit Health, 1 (2019), pp. e106-e107
[36]
Parekh VS, Jacobs MA. Radiomic synthesis using deep convolutional neural networks. arXiv:181011090. 2018:1–4.
[37]
T. Refaee, Z. Salahuddin, A.N. Frix, C. Yan, G. Wu, H.C. Woodruff, et al.
Diagnosis of idiopathic pulmonary fibrosis in high-resolution computed tomography scans using a combination of handcrafted radiomics and deep learning
Front Med (Lausanne), 23 (2022), Article 915243
[38]
X. Li, L. Yang, X. Jiao
Comparison of traditional radiomics, deep learning radiomics and fusion methods for axillary lymph node metastasis prediction in breast cancer
AcadRadiol, S1076–6332 (2022), pp. 00571-00572
[39]
HeK, Zhang X, RenS, Sun J. Deep Residual Learning for Image Recognition. In: IEEE Conference on Computer Vision and Pattern Recognition. IEEE; 2016.
[40]
Zagoruyko S, Komodakis N. Wide residual networks. arXiv preprint arXiv:1605.07146; 2016.
[41]
K.M. Kraus, M. Oreshko, D. Bernhardt, S.E. Combs, J.C. Peeken
Dosiomics and radiomics to predict pneumonitis after thoracic stereotactic body radiotherapy and immune checkpoint inhibition
Front Oncol, 15 (2023), Article 1124592
[42]
J. Wang, J. Cao, S. Yuan, W. Ji, D. Arenberg, J. Dai, et al.
Poor baseline pulmonary function may not increase the risk of radiation-induced lung toxicity
Int J Radiat Oncol Biol Phys, 85 (2013), pp. 798-804
[43]
D. Wang, J. Zhu, J. Sun, B. Li, Z. Wang, L. Wei, et al.
Functional and biologic metrics for predicting radiation pneumonitis in locally advanced non-small cell lung cancer patients treated with chemoradiotherapy
Clin Trans Oncol, 14 (2012), pp. 943-952
[44]
M. Yamada, S. Kudoh, K. Hirata, T. Nakajima, J. Yoshikawa
Risk factors of pneumonitis following chemoradiotherapy for lung cancer
Eur J Cancer, 34 (1998), pp. 71-75
[45]
W. Wang, Y. Xu, M. Schipper, M.M. Matuszak, T. Ritter, Y. Cao, et al.
Effect of normal lung definition on lung dosimetry and lung toxicity prediction in radiation therapy treatment planning
Int J Radiat Oncol Biol Phys, 86 (2013), pp. 956-963
[46]
M. Kim, J. Lee, B. Ha, R. Lee, K.J. Lee, H.S. Suh
Factors predicting radiation pneumonitis in locally advanced non-small cell lung cancer
Radiat Oncol J, 29 (2011), pp. 181-190
[47]
L.L. Shi, J.H. Yang, H.F. Yao
Multiple regression analysis of risk factors related to radiation pneumonitis
World J Clin Cases, 11 (2023), pp. 1040-1048
[48]
H. Yamashita, W. Takahashi, A. Haga, K. Nakagawa
Radiation pneumonitis after stereotactic radiation therapy for lung cancer
World J Radiol, 6 (2014), pp. 708-715
[49]
J.M. Luna, H.H. Chao, E.S. Diffenderfer, G. Valdes, C. Chinniah, G. Ma, et al.
Predicting radiation pneumonitis in locally advanced stage II-III non-small cell lung cancer using machine learning
Radiother Oncol, 133 (2019), pp. 106-112
[50]
T. Ullah, H. Patel, G.M. Pena, R. Shah, A.M. Fein
A contemporary review of radiation pneumonitis
Curr Opin Pulm Med, 26 (2020), pp. 321-325
[51]
H. Zhuang, H. Hou, Z. Yuan, J. Wang, Q. Pang, L. Zhao, et al.
Preliminary analysis of the risk factors for radiation pneumonitis in patients with non-small-cell lung cancer treated with concurrent erlotinib and thoracic radiotherapy
Onco Targets Ther, 24 (2014), pp. 807-813
[52]
S. Yilmaz, Y.G. Adas, A. Hicsonmez, M.N. Andrieu, S. Akyurek, S.C. Gokce
Evaluation of the radiation pneumonia development risk in lung cancer cases
Asian Pac J Cancer Prev, 15 (2014), pp. 7371-7375
[53]
E. Huynh, T.P. Coroller, V. Narayan, V. Agrawal, Y. Hou, J. Romano, et al.
CT-based radiomic analysis of stereotactic body radiation therapy patients with lung cancer
Radiother Oncol, 120 (2016), pp. 258-266
[54]
L. Zou, L. Chu, F. Xia, L. Zhou, X. Yang, J. Ni, et al.
Is clinical target volume necessary?-a failure pattern analysis in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy using intensity-modulated radiotherapy technique
Transl Lung Cancer Res, 9 (2020), pp. 1986-1995
[55]
F. Liu, B. Qiu, Y. Xi, Y. Luo, Q. Luo, Y. Wu, et al.
Efficacy of Thymosin α1 in management of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy: a phase 2 clinical trial (GASTO-1043)
Int J Radiat Oncol Biol Phys, 114 (2022), pp. 433-443
[56]
Y. Wang, Z.G. Liu, H. Yuan, W. Deng, J. Li, Y. Huang, et al.
The Reciprocity between Radiotherapy and Cancer Immunotherapy
Clin Cancer Res, 25 (2019), pp. 1709-1717
[57]
J.J. Luke, J.M. Lemons, T.G. Karrison, S.P. Pitroda, J.M. Melotek, Y. Zha, et al.
Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors
J Clin Oncol, 36 (2018), pp. 1611-1618
[58]
W.S.M.E. Theelen, H.M.U. Peulen, F. Lalezari, V. van der Noort, J.F. de Vries, J.G.J.V. Aerts, et al.
Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial
JAMA Oncol, 5 (2019), pp. 1276-1282
[59]
S. Tian, J.M. Switchenko, Z.S. Buchwald, P.R. Patel, J.W. Shelton, S.E. Kahn, et al.
Lung stereotactic body radiation therapy and concurrent immunotherapy: a multicenter safety and toxicity analysis
Int J Radiat Oncol Biol Phys, 108 (2020), pp. 304-313
[60]
F. Teng, M. Li, J. Yu
Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications
BMC Med, 18 (2020), p. 275
[61]
S.L. Tucker, T. Xu, H. Paganetti, T. Deist, V. Verma, N. Choi, et al.
Validation of effective dose as a better predictor of radiation pneumonitis risk than mean lung dose: secondary analysis of a randomized trial
Int J Radiat Oncol Biol Phys, 103 (2019), pp. 403-410
点击阅读原文
排版编辑:肿瘤资讯-Hanna











 苏公网安备32059002004080号
苏公网安备32059002004080号


