
以下内容原文发布于AACR官方博客《Cancer Research Catalyst》,中文内容仅做参考,请点击文末“阅读原文”,阅览原文内容。
Journal: Blood Cancer Discovery
Trisomy 8 Defines a Distinct Subtype of Myeloproliferative Neoplasms Driven by the MYC–Alarmin Axis
Despite advances in understanding the genetic abnormalities in myeloproliferative neoplasms (MPN) and the development of JAK2 inhibitors, there is an urgent need to devise new treatment strategies, particularly for patients with triple-negative (TN) myelofibrosis (MF) who lack mutations in the JAK2 kinase pathway and have very poor clinical outcomes. Here we report that MYC copy number gain and increased MYC expression frequently occur in TN-MF and that MYC-directed activation of S100A9, an alarmin protein that plays pivotal roles in inflammation and innate immunity, is necessary and sufficient to drive development and progression of MF. Notably, the MYC-S100A9 circuit provokes a complex network of inflammatory signaling that involves numerous hematopoietic cell types in the bone marrow microenvironment. Accordingly, genetic ablation of S100A9 or treatment with small molecules targeting the MYC-S100A9 pathway effectively ameliorates MF phenotypes, highlighting the MYC–alarmin axis as a novel therapeutic vulnerability for this subgroup of MPNs.
Significance: This study establishes that MYC expression is increased in TN-MPNs via trisomy 8, that a MYC-S100A9 circuit manifest in these cases is sufficient to provoke myelofibrosis and inflammation in diverse hematopoietic cell types in the BM niche, and that the MYC-S100A9 circuit is targetable in TN-MPNs.
Journal: Cancer Discovery
Activating point mutations in the MET tyrosine kinase domain (TKD) are oncogenic in a subset of papillary renal cell carcinomas. Here, using comprehensive genomic profiling among >600,000 patients, we identify activating MET TKD point mutations as putative oncogenic driver across diverse cancers, with a frequency of ∼0.5%. The most common mutations in the MET TKD defined as oncogenic or likely oncogenic according to OncoKB resulted in amino acid substitutions at positions H1094, L1195, F1200, D1228, Y1230, M1250, and others. Preclinical modeling of these alterations confirmed their oncogenic potential and also demonstrated differential patterns of sensitivity to type I and type II MET inhibitors. Two patients with metastatic lung adenocarcinoma harboring MET TKD mutations (H1094Y, F1200I) and no other known oncogenic drivers achieved confirmed partial responses to a type I MET inhibitor. Activating MET TKD mutations occur in multiple malignancies and may confer clinical sensitivity to currently available MET inhibitors.
Significance: The identification of targetable genomic subsets of cancer has revolutionized precision oncology and offers patients treatments with more selective and effective agents. Here, we demonstrate that activating, oncogenic MET tyrosine kinase domain mutations are found across a diversity of cancer types and are responsive to MET tyrosine kinase inhibitors.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Joint Associations of Diet and Device-Measured Physical Activity with Mortality and Incident CVD and Cancer: A Prospective Analysis of the UK Biobank Study
Background: We examined the joint associations of diet and device-measured intensity-specific physical activity (PA) with all-cause mortality (ACM), cardiovascular disease (CVD), and cancer incidence.
Methods: We used data from 79,988 participants from the UK Biobank, a population-based prospective cohort study. Light PA (LPA), moderate-to-vigorous PA (MVPA), vigorous PA (VPA), and total PA (TPA) were measured using a wrist-worn accelerometer. Diet quality score (DQS) was based on 10 foods and ranged from 0 (unhealthiest) to 100 (healthiest) points. We derived joint PA and diet variables. Outcomes were ACM, CVD, and cancer incidence including PA, diet and adiposity-related (PDAR) cancer.
Results: During a median follow-up of 8 years, 2,863 deaths occurred, 11,053 participants developed CVD, 7,005 developed cancer, and 3,400 developed PDAR cancer. Compared with the least favorable referent group (bottom PA tertile/low DQS), participants with middle and high (total and intensity specific) PA, except for LPA, had lower ACM risk and incident CVD risk, regardless of DQS. For example, among middle and high VPA and high DQS groups, CVD HR were 0.79 (95% CI, 0.74–0.86) and 0.75 (95% CI, 0.69–0.82), respectively. The pattern of cancer results was less pronounced but in agreement with the ACM and CVD incidence findings (e.g., HR, 0.90, 95% CI, 0.81–0.99; 0.88, 0.79–0.98; and 0.82, 0.74–0.92 among high VPA for low, moderate, and high DQS groups, respectively).
Conclusions: Device-measured PA reveals novel joint associations with diet on health outcomes.
Impact: Our results emphasize the crucial role of PA in addition to a healthy diet for reducing chronic diseases and mortality risk.
Journal: Cancer Immunology Research
T-Cell Expression of CXCL13 is Associated with Immunotherapy Response in a Sex-Dependent Manner in Patients with Lung Cancer
Emerging evidence in preclinical models demonstrates that antitumor immunity is not equivalent between males and females. However, more investigation in patients and across a wider range of cancer types is needed to fully understand sex as a variable in tumor immune responses. We investigated differences in T-cell responses between male and female patients with lung cancer by performing sex-based analysis of single cell transcriptomic datasets. We found that the transcript encoding CXC motif chemokine ligand 13 (CXCL13), which has recently been shown to correlate with T-cell tumor specificity, is expressed at greater levels in T cells isolated from female compared with male patients. Furthermore, increased CXCL13 expression was associated with response to PD1–targeting immunotherapy in female but not male patients. These findings suggest that there are sex-based differences in T-cell function required for response to anti–PD1 therapy in lung cancer that may need to be considered during patient treatment decisions.
Journal: Cancer Prevention Research
Colon Age: A Metric for Whether and How to Screen Male Veterans for Early-Onset Colorectal Cancer
We aimed to develop a metric for estimating risk for early-onset colorectal cancer (EOCRC) to help decide whether and how to screen persons < age 50. We used risk prediction models derived and validated on male veterans to calculate the RRs for six scenarios: one low-risk scenario (no risk factors present), four intermediate risk scenarios (some risk factors present), and one high-risk scenario (all risk factors present) for three age groups (35–39, 40–44, and 45–49 years). For each scenario, we estimated absolute colorectal cancer risk using Surveillance Epidemiology and End Results colorectal cancer incidence rates and each scenario’s RR. We identified the current Surveillance Epidemiology and End Results 5-year age group to which the revised estimate was closest and refer to the midpoint of this group as the “colon age.” When the revised estimate equals or exceeds that for 50- to 54-year-olds and for 70- to 74-year-olds, respective recommendations were made for (any) colorectal cancer screening and screening with colonoscopy. Among the scenarios, there was inconsistency between the two models for the 35 to 39 and 40 to 44 age groups, with only the 15-variable model recommending screening for the higher-risk 35- to 39-year-olds. Both models recommended screening for some intermediate risk and high-risk 40- to 44-year-olds. The models were well aligned on whether and how to screen most 45- to 49-year-olds. Using risk factors for EOCRC with colorectal cancer incidence rates, “colon age” may be useful for shared decision-making about whether and how to screen male veterans <50 years. For 45- to 49-year-olds, the 7-variable model may be preferred by patients, providers, and health systems.
Prevention Relevance: A new metric known as “colon age” expresses risk of EOCRC based on biological risk and may be useful for providers to explain and for patients to understand colorectal cancer risk when considering whether and how to be screened for colorectal cancer prior to age 45 or 50.
Journal: Cancer Research (August 1 issue)
Human 3D Ovarian Cancer Models Reveal Malignant Cell–Intrinsic and –Extrinsic Factors That Influence CAR T-cell Activity
In vitro preclinical testing of chimeric antigen receptor (CAR) T cells is mostly carried out in monolayer cell cultures. However, alternative strategies are needed to take into account the complexity and the effects of the tumor microenvironment. Here, we describe the modulation of CAR T-cell activity by malignant cells and fibroblasts in human three-dimensional (3D) in vitro cell models of increasing complexity. In models combining mucin-1 (MUC1) and TnMUC1 CAR T cells with human high-grade serous ovarian cancer cell spheroids, malignant cell–intrinsic resistance to CAR T-cell killing was due to defective death receptor signaling involving TNFα. Adding primary human fibroblasts to spheroids unexpectedly increased the ability of CAR T cells to kill resistant malignant cells as CCL2 produced by fibroblasts activated CCR2/4+ CAR T cells. However, culturing malignant cells and fibroblasts in collagen gels engendered production of a dense extracellular matrix that impeded CAR T-cell activity in a TGFβ-dependent manner. A vascularized microfluidic device was developed that allowed CAR T cells to flow through the vessels and penetrate the gels in a more physiological way, killing malignant cells in a TNFα-dependent manner. Complex 3D human cell models may provide an efficient way of screening multiple cytotoxic human immune cell constructs while also enabling evaluation of mechanisms of resistance involving cell–cell and cell–matrix interactions, thus accelerating preclinical research on cytotoxic immune cell therapies in solid tumors.
Significance: Three-dimensional in vitro models of increasing complexity uncover mechanisms of resistance to CAR T cells in solid tumors, which could help accelerate development of improved CAR T-cell constructs.
Journal: C ancer Research (August 15 issue)
Serine Depletion Promotes Antitumor Immunity by Activating Mitochondrial DNA-Mediated cGAS-STING Signaling
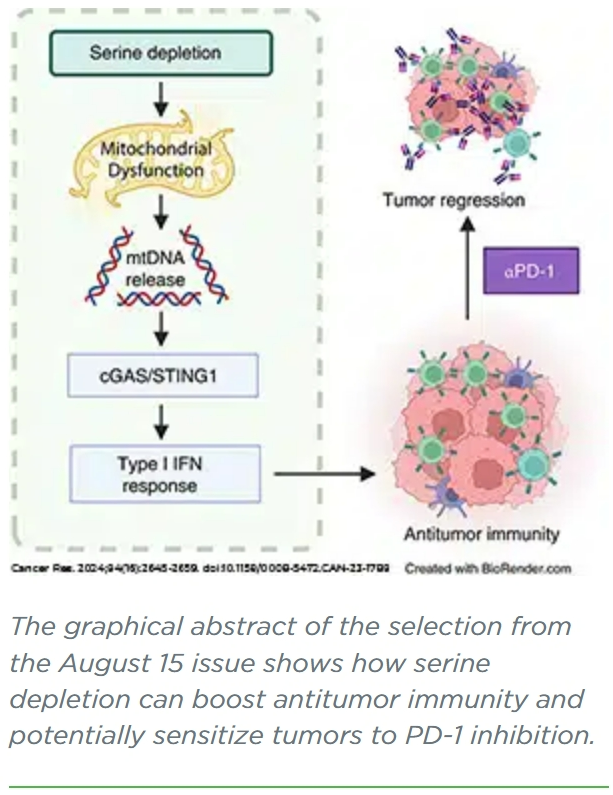
Serine is critical for supporting cancer metabolism, and depriving malignant cells of this nonessential amino acid exerts antineoplastic effects, in large part, through disrupting metabolic pathways. Given the intricate relationship between cancer metabolism and the immune system, the metabolic defects imposed by serine deprivation might impact tumor-targeting immunity. In this study, we demonstrated that restricting endogenous and exogenous sources of serine in colorectal cancer cells results in mitochondrial dysfunction, leading to mitochondrial DNA (mtDNA) accumulation in the cytosol and consequent cGAS-STING1-driven type I IFN secretion. Depleting mtDNA or blocking its release attenuated cGAS-STING1 activation during serine deprivation. In vivo studies revealed that serine deprivation limits tumor growth, accompanied by enhanced type I IFN signaling and intratumoral infiltration of immune effector cells. Notably, the tumor-suppressive and immune-enhancing effects of serine restriction were impaired by T-cell depletion and IFN receptor blockade. Moreover, disrupting cGAS-STING1 signaling in colorectal cancer cells limited the immunostimulatory and tumor-suppressive effects of serine deprivation. Lastly, serine depletion increased the sensitivity of tumors to an immune checkpoint inhibitor targeting PD-1. Taken together, these findings reveal a role for serine as a suppressor of antitumor immunity, suggesting that serine deprivation may be employed to enhance tumor immunogenicity and improve responsiveness to immune checkpoint inhibitors.
Significance: Depriving cancer cells of serine provokes mitochondrial perturbations that induce cytosolic mitochondrial DNA accumulation and subsequent activation of cGAS-STING signaling, stimulating tumor-targeting immune responses that can be enhanced with PD-1 targeted therapy.
Journal: C linical Cancer Research (August 1 issue)
Plasma versus Tissue Tumor Mutational Burden as Biomarkers of Durvalumab plus Tremelimumab Response in Patients with Metastatic Colorectal Cancer in the CO.26 Trial
Purpose: Tissue-derived tumor mutation burden (TMB) of ≥10 mutations/Mb is a histology-agnostic biomarker for the immune checkpoint inhibitor (ICI) pembrolizumab. However, the dataset in which this was validated lacked colorectal cancers (CRC), and there is limited evidence for immunotherapy benefits in CRC using this threshold.
Patients and Methods: CO.26 was a randomized phase II study of 180 patients, comparing durvalumab and tremelimumab (D + T, n = 119 patients) versus best supportive care (BSC; n = 61 patients). ctDNA sequencing was available for 168 patients (n = 118 D + T; n = 50), of whom 165 had evaluable plasma TMB (pTMB). Tissue sequencing was available for 108 patients. Optimal thresholds for stratifying patients based on OS were determined using a minimal P value approach. This report includes the final OS analysis.
Results: Tissue TMB ≥10 mutations/Mb was not predictive of benefit from D + T compared with BSC in microsatellite stable (MSS) metastatic CRC [HR, 0.71 (95% CI, 0.28–1.80); P = 0.47]. No tissue TMB threshold could identify a high TMB group that benefited from ICI. By contrast, plasma TMB (pTMB) ≥28 mutations/Mb was predictive of benefit from D + T [HR, 0.34 (95% CI, 0.13–0.85); P = 0.022], as was clonal pTMB ≥10.6 mutations/Mb [HR, 0.10 (95% CI, 0.014–0.79); P = 0.029] and subclonal pTMB ≥25.9/Mb [HR, 0.20 (95% CI, 0.061–0.69); P = 0.010]. Higher pTMB was associated with length of time on cytotoxic agents (P = 0.021) and prior anti-EGFR exposure (P = 2.44 × 10−06).
Conclusions: pTMB derived from either clonal or subclonal mutations may identify a group likely to benefit from immunotherapy, although validation is required. Tissue TMB provided no predictive utility for immunotherapy in this trial.
Journal: Clinical Cancer Research (August 15 issue)
Nivolumab with or without Ipilimumab Combined with Stereotactic Body Radiotherapy in Patients with Metastatic Biliary Tract Cancer: A Randomized Phase 2 Study
Purpose: The purpose of this study was to evaluate the clinical benefits of nivolumab with/without ipilimumab combined with stereotactic body radiotherapy (SBRT) in patients with pretreated metastatic biliary tract cancer (mBTC).
Patients and Methods: The study was a phase 2 randomized trial with Simon’s optimal two-stage design requiring 36 evaluable patients per group after second stage. Sixty-one patients were included from September 2018 to January 2022 and randomized (1:1) to receive SBRT (15 Gy × 1 on day 1 to a primary or metastatic lesion) and nivolumab (3 mg/kg intravenously on day 1 and every 2 weeks) with/without ipilimumab (1 mg/kg intravenously on day 1 and every 6 weeks). Primary endpoint was clinical benefit rate (CBR), defined as the percentage of patients with complete response, partial response, or stable disease. Decision to continue accrual into the second stage depended on the CBR from the first stage.
Results: Forty-two patients received SBRT/nivolumab/ipilimumab with a CBR of 31.0% [95% confidence interval (CI), 17.6–47.1]. Five patients (11.9%) achieved partial response with median duration of 4.4 months (range, 1.1–21.5). Nineteen patients received SBRT/nivolumab. This group was closed after the initial stage based on a CBR of 10.5% (95% CI, 1.3–33.1). Adverse events were graded with National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Grade ≥3 treatment-related adverse events occurred in 13 (31%) and 3 (16%) patients in the SBRT/nivolumab/ipilimumab and SBRT/nivolumab groups, respectively. One patient died from immune-related hepatitis in the SBRT/nivolumab/ipilimumab group.
Conclusions: Combining SBRT, nivolumab, and ipilimumab is well tolerated, feasible, and shows response in a subgroup of patients with mBTC.
Journal: Molecular Cancer Research
DROSHA Regulates Mesenchymal Gene Expression in Wilms Tumor
Wilms tumor, the most common pediatric kidney cancer, resembles embryonic renal progenitors. Currently, there are no ways to therapeutically target Wilms tumor driver mutations, such as in the microRNA processing gene DROSHA. In this study, we used a “multiomics” approach to define the effects of DROSHA mutation in Wilms tumor. We categorized Wilms tumor mutations into four mutational subclasses with unique transcriptional effects: microRNA processing, MYCN activation, chromatin remodeling, and kidney developmental factors. In particular, we find that DROSHA mutations are correlated with de-repressing microRNA target genes that regulate differentiation and proliferation and a self-renewing, mesenchymal state. We model these findings by inhibiting DROSHA expression in a Wilms tumor cell line, which led to upregulation of the cell cycle regulator cyclin D2 (CCND2). Furthermore, we observed that DROSHA mutations in Wilms tumor and DROSHA silencing in vitro were associated with a mesenchymal state with aberrations in redox metabolism. Accordingly, we demonstrate that Wilms tumor cells lacking microRNAs are sensitized to ferroptotic cell death through inhibition of glutathione peroxidase 4, the enzyme that detoxifies lipid peroxides.
Implications: This study reveals genotype–transcriptome relationships in Wilms tumor and points to ferroptosis as a potentially therapeutic vulnerability in one subset of Wilms tumor.
Jour nal: Molecular Cancer Therapeutics
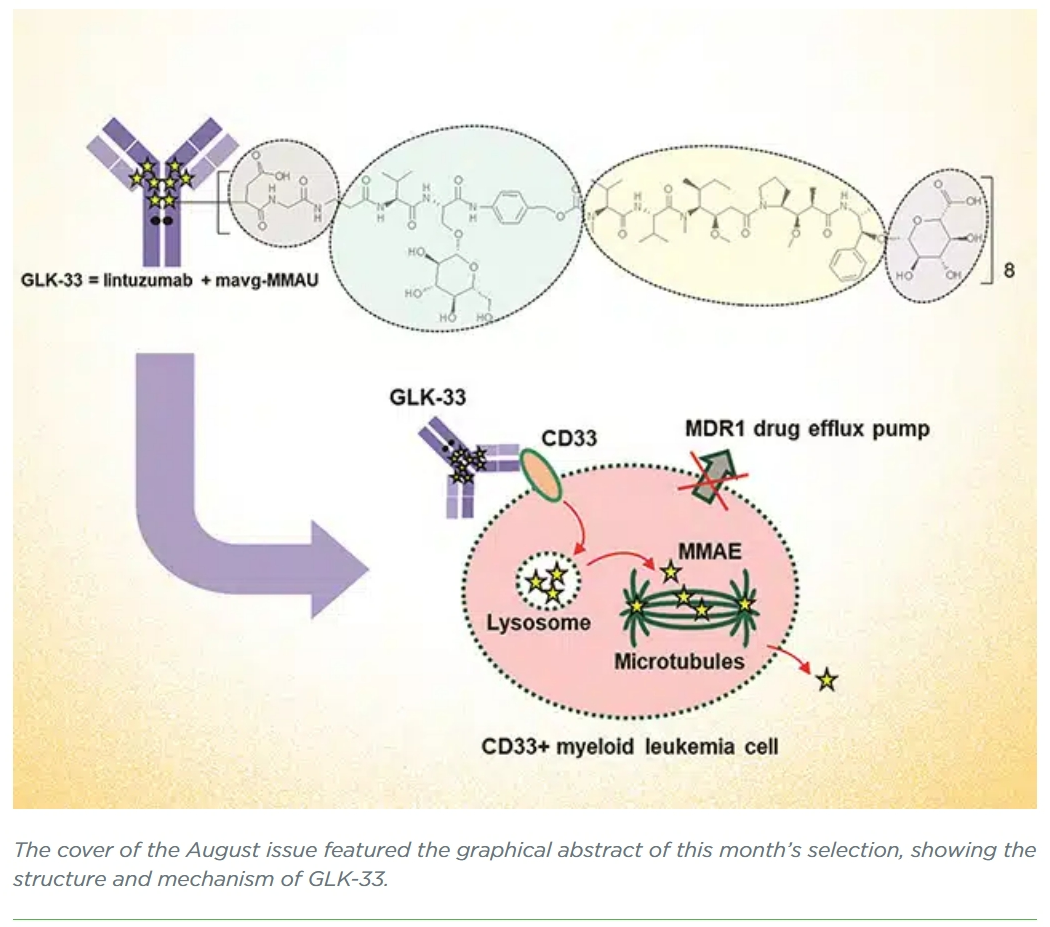
Targeting CD33+ Acute Myeloid Leukemia with GLK-33, a Lintuzumab–Auristatin Conjugate with a Wide Therapeutic Window
CD33 (Siglec-3) is a cell surface receptor expressed in approximately 90% of acute myeloid leukemia (AML) blasts, making it an attractive target for therapy of AML. Although previous CD33-targeting antibody–drug conjugates (ADC) like gemtuzumab ozogamicin (GO, Mylotarg) have shown efficacy in AML treatment, they have suffered from toxicity and narrow therapeutic window. This study aimed to develop a novel ADC with improved tolerability and a wider therapeutic window. GLK-33 consists of the anti-CD33 antibody lintuzumab and eight mavg-MMAU auristatin linker payloads per antibody. The experimental methods included testing in cell cultures, patient-derived samples, mouse xenograft models, and rat toxicology studies. GLK-33 exhibited remarkable efficacy in reducing cell viability within CD33-positive leukemia cell lines and primary AML samples. Notably, GLK-33 demonstrated antitumor activity at single dose as low as 300 mg/kg in mice, while maintaining tolerability at single dose of 20 to 30 mg/kg in rats. In contrast with both GO and lintuzumab vedotin, GLK-33 exhibited a wide therapeutic window and activity against multidrug-resistant cells. The development of GLK-33 addresses the limitations of previous ADCs, offering a wider therapeutic window, improved tolerability, and activity against drug-resistant leukemia cells. These findings encourage further exploration of GLK-33 in AML through clinical trials.
Journal: Cancer Research Communications
The Effect of Exposure to Neighborhood Violence on Glucocorticoid Receptor Signaling in Lung Tumors
Despite lower rates and intensity of smoking, Black men experience a higher incidence of lung cancer compared to white men. The racial disparity in lung cancer is particularly pronounced in Chicago, a highly segregated urban city. Neighborhood conditions, particularly social stress, may play a role in lung tumorigenesis. Preliminary studies indicate that Black men residing in neighborhoods with higher rates of violent crime have significantly higher levels of hair cortisol, an indicator of stress response. To examine the relationship between social stress exposure and gene expression in lung tumors, we investigated glucocorticoid receptor (GR) binding in 15 lung tumor samples in relation to GR target gene expression levels and zip code level residential violent crime rates. Spatial transcriptomics and a version of ChIP sequencing known as CUT&RUN were used. Heatmap of genes, pathway analysis, and motif analysis were conducted at the statistical significance of P < 0.05. GR recruitment to chromatin was correlated with zip code level residential violent crime rate and overall GR binding increased with higher violent crime rates. Our findings suggest that exposure to residential violent crime may influence tumor biology via reprogramming GR recruitment. Prioritizing lung cancer screening in neighborhoods with increased social stress, such as high levels of violent crime, may reduce racial disparities in lung cancer.
Significance: Exposure to neighborhood violent crime is correlated with glucocorticoid signaling and lung tumor gene expression changes associated with increased tumor aggressiveness, suggesting social conditions ha ve downstream biophysical consequences that contribute to lung cancer disparities.
更多内容,请点击“阅读原文”
排版编辑:肿瘤资讯-Astrid











 苏公网安备32059002004080号
苏公网安备32059002004080号


