整理:肿瘤资讯
专家介绍
上海交通大学医学院附属仁济医院胃肠外科行政副主任
仁济医院-杭州湾医院普外科科主任
仁济医院结直肠肿瘤多学科团队首席专家
中国医师协会结直肠肿瘤专业委员会常委
中华医学会外科分会腹腔镜与内镜外科学组委员
中国医疗保健国际交流促进会外科分会常委
中华医学会医疗鉴定专家库成员
中国成人教育协会消化外科委员会常委
国家结直肠肿瘤质控专家委员会委员
中国医学装备协会腔镜与微创技术分会副会长
国家卫健委能力建设和继续教育外科学委员
中国NOSES联盟上海分会副理事长
中国医师协会外科分会结直肠专业委员会委员
海峡两岸医药卫生交流协会消化道外科常委
中国抗癌协会大肠癌专业委员会常委
上海市抗癌协会胃肠肿瘤腹腔镜专业副主任委员
上海市抗癌协会大肠癌专业委员会副主任委员
上海市医学会外科分会结直肠专业学组副主任委员
上海市医学会普外科专科分会委员
《中华胃肠外科杂志》编辑委员
《腹腔镜外科杂志》编辑委员
PIK3CA基因突变在结直肠癌中的比例与病理特征
PIK3CA突变是癌症中最常见的体细胞突变之一1,PIK3CA基因编码IA类磷脂酰肌醇3-激酶(PI3K)的催化亚基p110α2。根据Cancer Genome Atlas和美国国家癌症研究所的数据,PIK3CA是几种主要癌症类型中突变率第二高的基因,突变频率超过12%3-4。不同研究中的突变频率数据有所不同,其中在结直肠癌的突变频率为32%5。
PIK3CA突变存在三个突变位点,E542K、E545K和H1047R6,这些获得性功能突变导致PI3K活性增加,并且在体内具有致癌性7。PI3K/Akt信号转导在结直肠癌和其他癌症的发展中起着重要作用8。PIK3CA突变导致细胞增殖增加、细胞凋亡减少和肿瘤侵袭7。多项研究报告称,PIK3CA突变患者的总体复发和转移率较高,生存率较低9-10。
体内和体外研究表明,该基因突变与癌症患者预后不良以及对化疗和单克隆抗体疗法等标准治疗产生耐药性有关11-12。因此,PIK3CA突变状态已被建议作为阿司匹林治疗结直肠癌的疗效预测因素13-14。
阿司匹林在PIK3CA突变结直肠癌患者中的辅助治疗:机制与临床应用
1. 作用机制
阿司匹林是已知的预防多种癌症(尤其是结直肠癌)的保护因子15,其分子机制尚不完全清楚。阿司匹林对结直肠肿瘤发生抑制作用的机制包括:一是通过各种细胞内通路直接作用于肿瘤细胞;二是通过抑制肿瘤微环境中的血小板以间接方式发挥抗癌作用。阿司匹林对PGE2的抑制作用将通过WNT信号通路、EGFR/PIK3CA/AKT/PPARδ、Ras/Raf/MAP激酶(MEK)/ERK这些通路调节结直肠肿瘤的发生;阿司匹林也作用于NFkB通路、AMPK/mTOR通路、凋亡通路这些细胞内通路发挥作用,与PGE2抑制无关16。此外,阿司匹林可以通过抑制血小板COX-1,进而抑制上皮细胞中COX-2的表达,这是阿司匹林抑制血小板介导的肿瘤发生机制之一17。
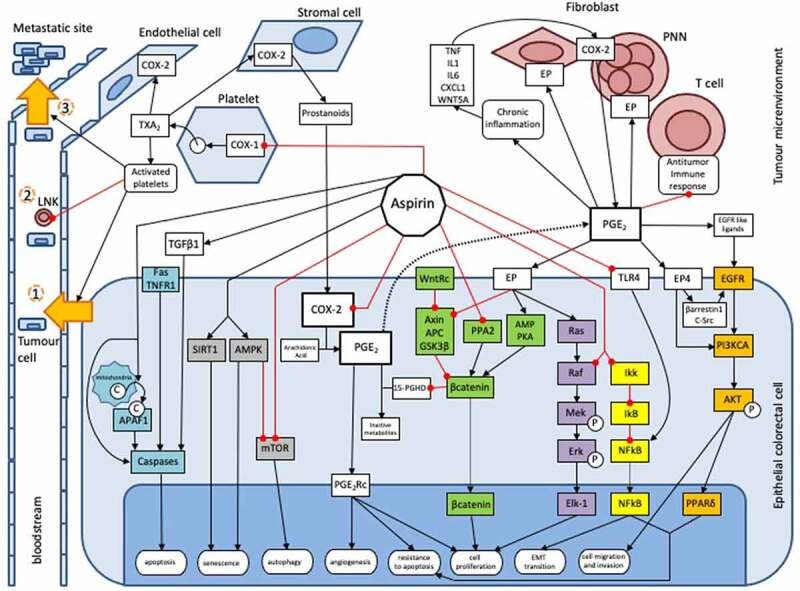
2. 研究进展
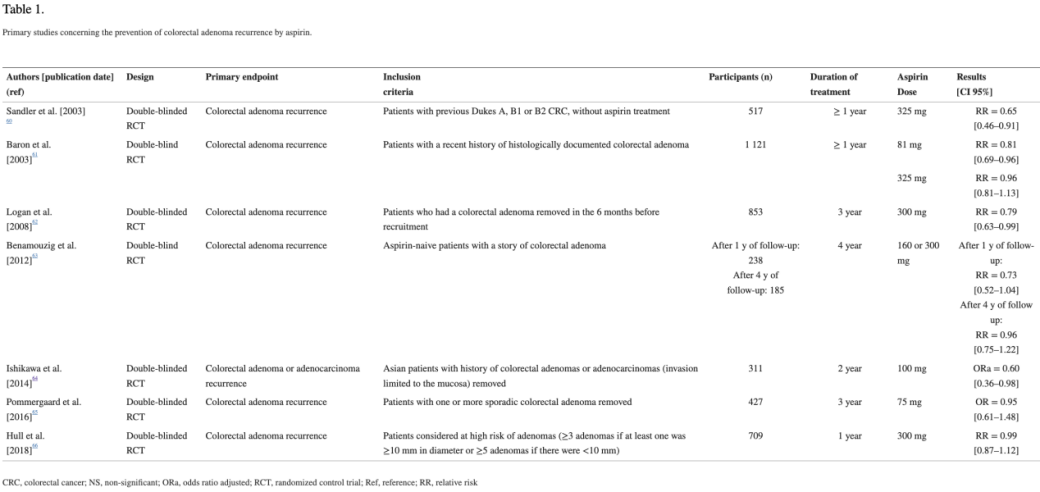
多项研究结果表明18-24:每日服用低剂量阿司匹林可降低结肠直肠腺瘤复发的风险,但吸烟者和高风险患者亚群中,这种益处似乎会降低。对于这些高风险亚群,有必要开展进一步的试验,评估具体的实验条件(如剂量和治疗持续时间)和终点。
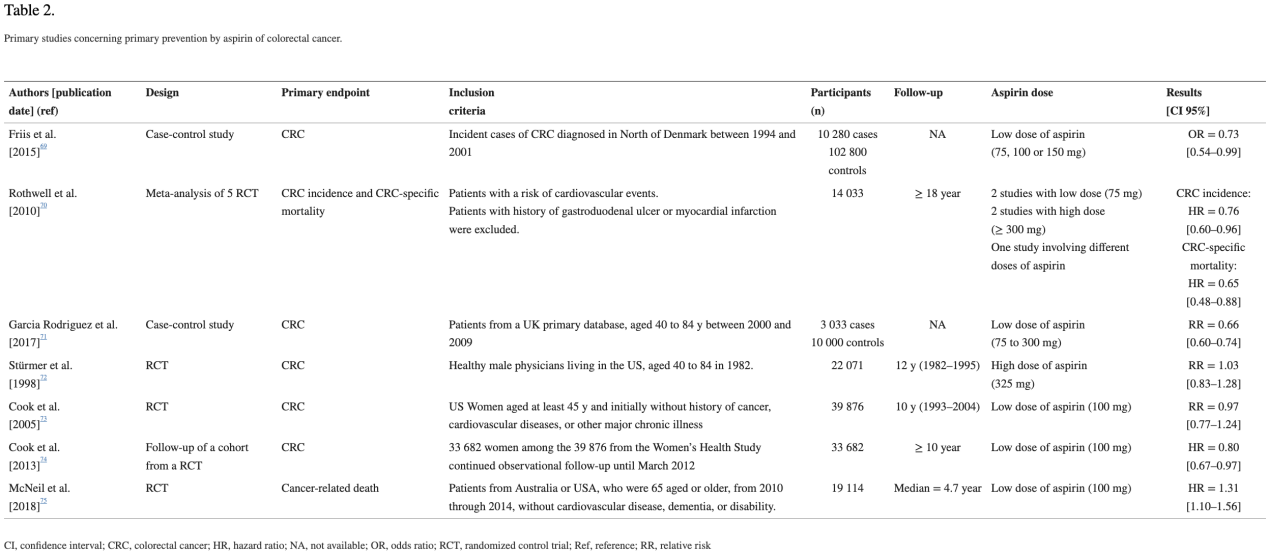
多项研究25-31表明,低剂量阿司匹林可降低50岁以上且心血管风险较高的人群的结直肠癌长期风险(超过10年)及其相关死亡率。目前,对于50至59岁且10年心血管事件风险大于10%的患者,建议使用阿司匹林进行心血管事件和结直肠癌的一级预防16。
也有证据表明,在结直肠癌发生后,阿司匹林具有重要意义32。除了对肿瘤进展和降期的影响外,一些研究表明,结直肠癌诊断后使用阿司匹林与结直肠癌特异性32-35和总体死亡率降低有关32,35-37。
COX-2抑制剂在PIK3CA突变结直肠癌患者中的辅助治疗:机制与临床应用
1. 作用机制
COX-2抑制剂对结直肠肿瘤发生抑制作用的机制包括:一是COX-2抑制剂可以显著抑制肿瘤患者体内前列腺素E2(PGE2)的产生,从而减轻炎症反应38;二是COX-2抑制剂通过调节胸苷激酶1(TK1)的表达,诱导结直肠癌细胞周期阻滞,抑制肿瘤细胞增殖39;COX-2抑制剂可能通过抑制血管生成相关因子的表达,抑制肿瘤血管形成40。
2. 研究进展
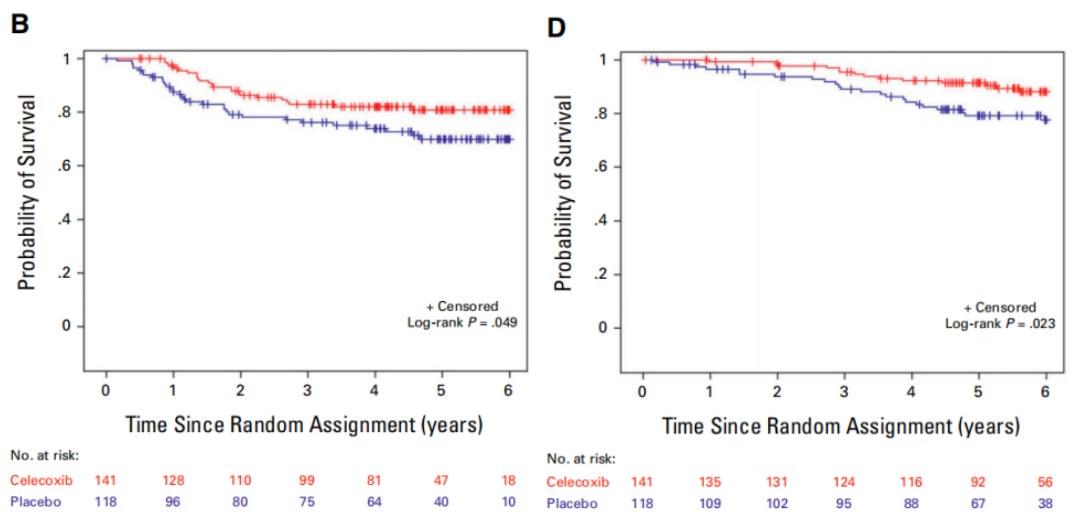
阿司匹林和非甾体抗炎药(NSAIDs)长期以来一直被研究为可能影响癌症发生和进展的药物41-42。在观察性研究和随机试验中,阿司匹林和环氧化酶-2(也称为COX-2)抑制剂与结直肠腺瘤和结直肠癌发病的低风险相关43-45。观察性研究表明,在结直肠癌诊断之前或之后使用阿司匹林或COX-2抑制剂与未使用相比,与疾病复发风险较低相关46-48。为了确定添加COX-2抑制剂塞来昔布是否能降低复发风险并改善结肠癌患者的生存,美国国家癌症研究所(NCI)赞助了CALGB/SWOG 80702(C80702)试验,CALGB/SWOG 80702研究46结果显示,对于PIK3CA突变的III期结肠癌患者,在标准化疗的基础上加用辅助塞来昔布治疗可以改善无病生存期(DFS)和总生存期(OS),可见COX-2抑制剂可提高PIK3CA突变III期结肠癌患者的生存率,这项研究为PIK3CA突变结肠癌患者的个体化治疗提供了新的证据和方向,有望改善这类患者的预后。
其他相关进展
一项荟萃分析报告称,肿瘤组织中COX-2的表达与诊断后服用阿司匹林者的死亡风险降低显著相关33;还有研究也表明,与未使用者相比,服用阿司匹林者的COX-2表达与总体生存率提高有关50。前列腺素代谢物(PGE-M)也可能是一种生物标志物,因为多项研究发现晚期和/或多发性结直肠腺瘤与较高水平的PGE-M显著相关51-52。
总结
PIK3CA基因突变在结直肠癌中的高频率和其对肿瘤生物学行为的显著影响,使其成为精准治疗的重要靶点。阿司匹林和COX-2抑制剂在PIK3CA突变结直肠癌中的应用,展示了其在降低肿瘤复发风险和提高患者生存率方面的潜力。阿司匹林通过多种细胞内通路和肿瘤微环境的调节,发挥其抗癌作用,而COX-2抑制剂则通过抑制前列腺素E2的产生和调节细胞周期等机制,抑制肿瘤进展。多项研究和临床试验的结果支持了这两类药物在PIK3CA突变结直肠癌中的辅助治疗作用。
未来的研究应进一步明确阿司匹林和COX-2抑制剂在不同患者亚群中的具体疗效,并探索其最佳使用剂量和治疗持续时间。此外,结合其他分子靶向治疗和免疫疗法,可能会为PIK3CA突变结直肠癌患者提供更加个体化和有效的治疗方案。这些进展在为结直肠癌的治疗带来了新的希望的同时,也为其他类型癌症的精准治疗提供了宝贵的经验和借鉴。
1. Hall DCN, Benndorf RA. Aspirin sensitivity of PIK3CA-mutated Colorectal Cancer: potential mechanisms revisited. Cell Mol Life Sci. 2022;79(7):393.
2. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606-619.
3. National Cancer Institute, Genomic Data Commons Data Portal, National Institute of Health. Distribution of most frequently mutated genes.
4. Kandoth C, Mclellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature.2013;502(7471):333–338.
5. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science (NewYork, N.Y.) 2004;304:554.
6. Liu S, Knapp S, Ahmed AA. The structural basis of PI3K cancer mutations: from mechanism to therapy. Cancer Res. 2014 Feb 1;74(3):641-6.
7. Samuels Y, Diaz LA Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7(6):561-573.
8. Papadatos-Pastos D, Rabbie R, Ross P, Sarker D. The role of the PI3K pathway in colorectal cancer. Crit Rev Oncol Hematol. 2015;94(1):18-30.
9. Ogino S, Nosho K, Kirkner GJ, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27(9):1477-1484.
10. Malinowsky K, Nitsche U, Janssen KP, et al. Activation of the PI3K/AKT pathway correlates with prognosis in stage II colon cancer. Br J Cancer. 2014;110(8):2081-2089.
11. Barault L, Veyrie N, Jooste V et al (2008) Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a populationbased series of colon cancers. Int J Cancer 122:2255–2259.
12. Malinowsky K, Nitsche U, Janssen K-P et al (2014) Activation of the PI3K/AKT pathway correlates with prognosis in stage II colon cancer. Br J Cancer 110:2081–2089.
13. Liao X, Lochhead P, Nishihara R et al (2012) Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 367:1596–1606.
14. Zumwalt TJ, Wodarz D, Komarova NL et al (2017) Aspirininduced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prevent Res (Philadelphia, Pa.) 10:208–218.
15. Qiao Y, Yang T, Gan Y, et al. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer. 2018;18(1):288.
16. Grancher A, Michel P, Di Fiore F, Sefrioui D. Colorectal cancer chemoprevention: is aspirin still in the game?. Cancer Biol Ther. 2022;23(1):446-461.
17. Dovizio M, Bruno A, Tacconelli S, Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013;191:39-65.
18. Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890.
19. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899.
20. Logan RFA, Grainge MJ, Shepherd VC, et al. Aspirin and folic acid for the prevention of recurrent colorectal adenomas.Gastroenterology. 2008;134 (1):29–38.
21. Benamouzig R, Uzzan B, Deyra J, et al. Prevention by dailysoluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. 2012;61(2):255–261.
22. Ishikawa H, Mutoh M, Suzuki S, et al. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut. 2014;63(11):1755–1759.
23. Pommergaard H-C, Burcharth J, Rosenberg J, et al. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. 2016;150 (1):114–122.e4.
24. Hull MA, Sprange K, Hepburn T, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebocontrolled, 2×2 factorial trial. Lancet. 2018;392 (10164):2583–2594.
25. Friis S, Riis AH, Erichsen R, et al. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med. 2015;163(5):347–355.
26. Rothwell PM, Wilson M, Elwin C-E, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750.
27. García Rodríguez LA, Soriano-Gabarró M, Bromley S, et al. New use of low-dose aspirin and risk of colorectal cancer by stage at diagnosis: a nested case–control study in UK general practice. BMC Cancer. 2017;17(1).
28. Stürmer T, Glynn RJ, Lee IM, et al. Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians’ Health Study. Ann Intern Med. 1998;128(9):713–720.
29. Cook NR, Lee I-M, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55.
30. Cook NR, Lee I-M, Zhang SM, et al. Alternateday, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85.
31. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379 (16):1519–1528.
32. Chan AT. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658.
33. Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64 (9):1419–1425.
34. Bastiaannet E, Sampieri K, Dekkers OM, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106(9):1564–1570.
35. McCowan C, Munro AJ, Donnan PT, et al. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer. 2013;49(5):1049–1057.
36. Goh CH, Goh HH, Leong WQ, et al. Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer. Anticancer Res. 2014;34(12):7407–7414.
37. Bains SJ, Mahic M, Myklebust TÅ, et al. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. J Clin Oncol. 2016;34(21):2501–2508.
38. Resler AJ, Makar KW, Heath L, et al. Genetic variation in prostaglandin synthesis and related pathways, NSAID use and colorectal cancer risk in the Colon Cancer Family Registry. Carcinogenesis. 2014;35(9):2121–2126.
39. Hubner RA, Muir KR, Liu J-F, et al. Ornithine decarboxylase G316A genotype is prognostic for colorectal adenoma recurrence and predicts efficacy of aspirin chemoprevention. Clin Cancer Res. 2008;14(8):2303–2309.
40. Barry EL, Mott LA, Sandler RS, et al. Variants downstream of the ornithine decarboxylase gene influence risk of colorectal adenoma and Aspirin Chemoprevention. Cancer Prev Res (Phila). 2011;4(12):2072–2082.
41. Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila). 2012;5(2):164-178.
42. Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342(26):1960-1968.
43. Bertagnolli MM. Cox-2 and cancer chemoprevention: picking up the pieces. Recent Results Cancer Res. 2007;174:73-78.
44. Chan AT. Aspirin, non-steroidal anti-inflammatory drugs and colorectal neoplasia: future challenges in chemoprevention. Cancer Causes Control. 2003;14(5):413-418.
45. Cross JT, Poole EM, Ulrich CM. A review of gene-drug interactions for nonsteroidal anti-inflammatory drug use in preventing colorectal neoplasia. Pharmacogenomics J. 2008;8(4):237-247.
46. Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649-658.
47. Ng K, Meyerhardt JA, Chan AT, et al. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst. 2014;107(1):345.
48. Coghill AE, Newcomb PA, Campbell PT, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60(4):491-498.
49. Nowak JA, Twombly T, Ma C, et al. Improved Survival With Adjuvant Cyclooxygenase 2 Inhibition in PIK3CA-Activated Stage III Colon Cancer: CALGB/SWOG 80702 (Alliance). J Clin Oncol. Published online June 18, 2024.
50. Gray RT, Cantwell MM, Coleman HG, et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin Transl Gastroenterol. 2017;8(4):e91.
51. Shrubsole MJ, Cai Q, Wen W, et al. Urinary prostaglandin E2 metabolite and risk for colorectal adenoma. Cancer Prev Res (Phila). 2012;5 (2):336–342. doi:10.1158/1940-6207.CAPR-11-0426.
52. Bezawada N, Song M, Wu K, et al. Urinary PGE-M levels are associated with risk of colorectal adenomas and chemopreventive response to anti-inflammatory drugs. Cancer Prev Res (Phila). 2014;7(7):758–765.
53. Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606.
54. Domingo E, Church DN, Sieber O, et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31(34):4297–4305.
55. Reimers MS, Bastiaannet E, Langley RE, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174(5):732–739.
排版编辑:栗子











 苏公网安备32059002004080号
苏公网安备32059002004080号


