免疫检查点是人体平衡免疫功能的两类分子,一类负责刺激免疫细胞,另一类负责抑制免疫细胞,后者包括免疫细胞程序性死亡蛋白 PD-1 及其配体 PD-L1 。肿瘤细胞能够利用PD-1和PD-L1抑制免疫细胞,让免疫细胞进入自杀程序,避免自己被免疫细胞消灭,而 PD-1或PD-L1抑制剂可以恢复免疫细胞抗肿瘤功能,已被大量随机对照试验证实对晚期乳腺癌免疫治疗有效。不过,对于早期乳腺癌免疫治疗,现有免疫检查点抑制剂随机对照试验结果有好有坏。由于免疫检查点抑制剂相当昂贵,而且存在一定毒性反应,故有必要对这些研究进行汇总分析,看看免疫检查点抑制剂对于早期乳腺癌免疫治疗有效性和安全性究竟如何。
2024年8月29日,《美国医学会杂志》肿瘤学分册在线发表 西班牙癌症研究协作组、巴塞罗那大学、希伯伦谷医院肿瘤研究所、奥古斯特皮桑耶尔生物医学研究院、巴塞罗那医院、巴塞罗那大学、凯龙肿瘤研究院、马德里大学国庆日医院、瑞典卡罗林大学、卡罗琳斯卡大学医院、法国巴黎萨克雷大学古斯塔夫鲁西研究院 的随机对照试验系统回顾和荟萃分析,总结了化疗±免疫检查点抑制剂对于不同类型早期乳腺癌患者的病理完全缓解、生存获益以及特定不良事件发生率。
该研究首先于2023年12月10日对美国国家医学图书馆PubMed数据库进行文献检索,确定全部可能符合条件的早期乳腺癌免疫检查点抑制剂联合化疗随机对照试验,由2位审阅者各自提取符合条件的随机对照试验数据,随后对提取的患者个体数据进行荟萃分析和试验水平随机效应荟萃分析。主要结局指标包括病理完全缓解、无事件生存以及不良事件。利用分层多因素比例风险回归模型对其他影响因素进行校正后比较化疗±免疫检查点抑制剂的风险比。
结果,9项随机对照试验符合分析标准,包括5114例患者,其中:
三阴性乳腺癌:2097例
激素受体阳性HER2阴性乳腺癌:1924例
HER2阳性乳腺癌:1115例
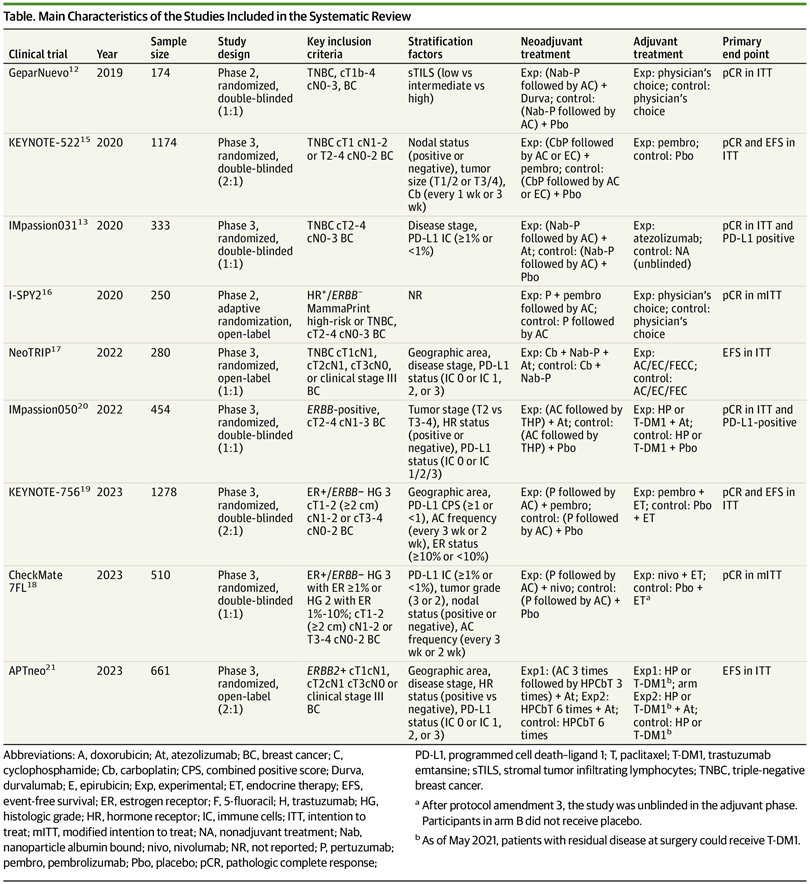
化疗 ±免疫检查点抑制剂
相比:
三阴性乳腺癌 :无论PD-L1是否阳性,病理完全缓解率显著较高(超过>10个百分点)
激素受体阳性HER2阴性乳腺癌 :仅 PD-L1阳性的病理完全缓解率显著较高(提高12.2个百分点)
HER2阳性乳腺癌: 未见显著获益
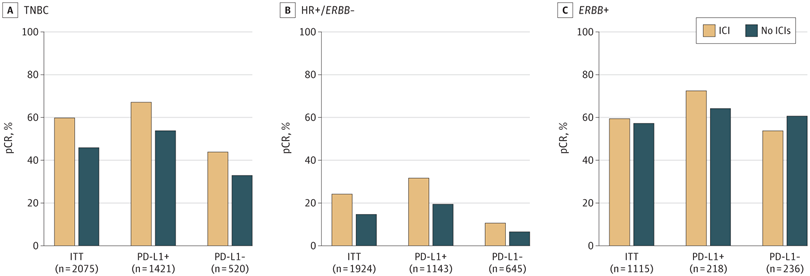
对于三阴性乳腺癌患者,化疗±免疫检查点抑制剂相比:
获得病理完全缓解:无事件生存率显著较高(5年无事件生存率:92.0%比88.0%,风险比:0.65,95%置信区间:0.42~1.00)
未获病理完全缓解:无事件生存率显著较高(5年无事件生存率:63.3%比56.1%,风险比:0.77,95%置信区间:0.61~0.98)
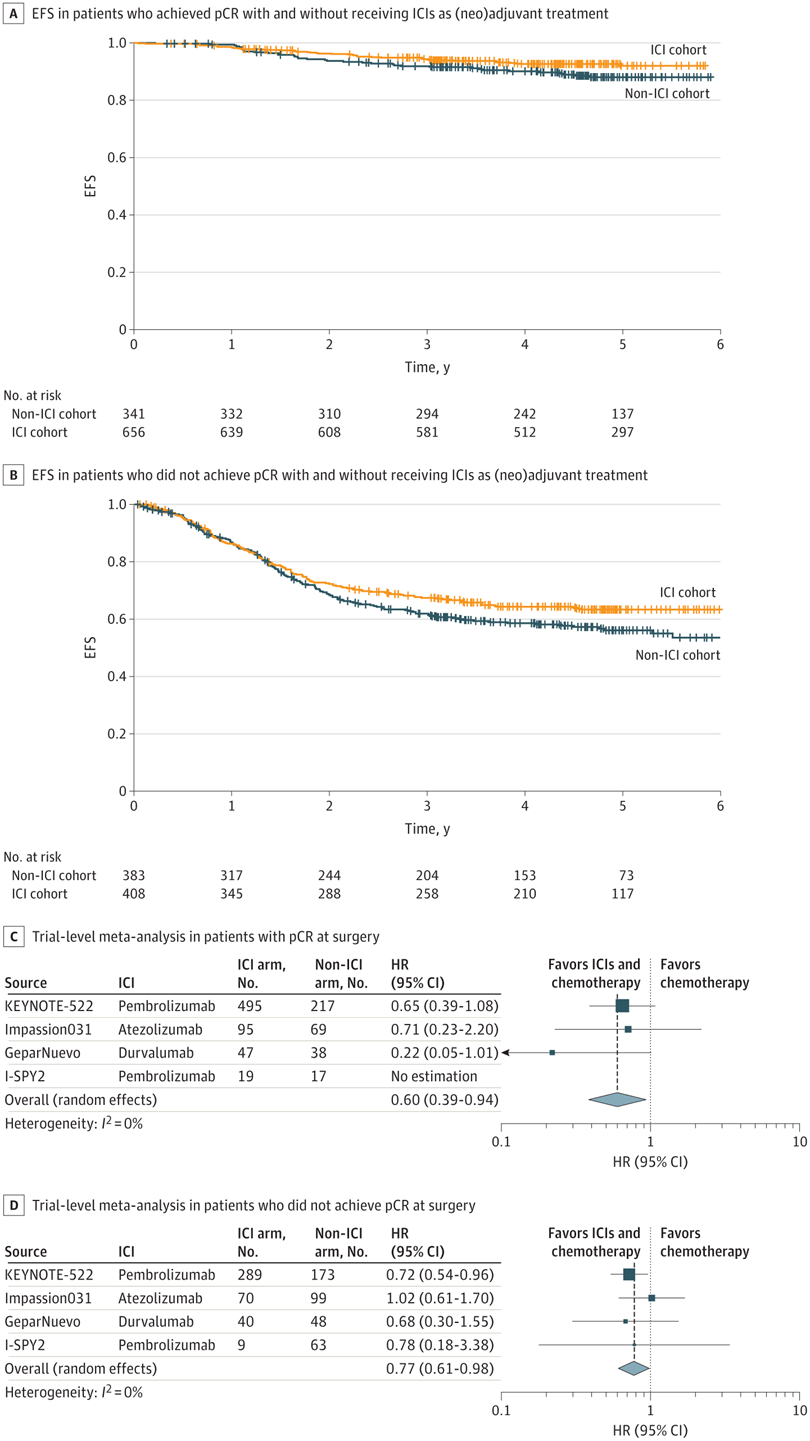
无论是否病理完全缓解,术后免疫检查点抑制剂均未获益,全部风险比都大于1。
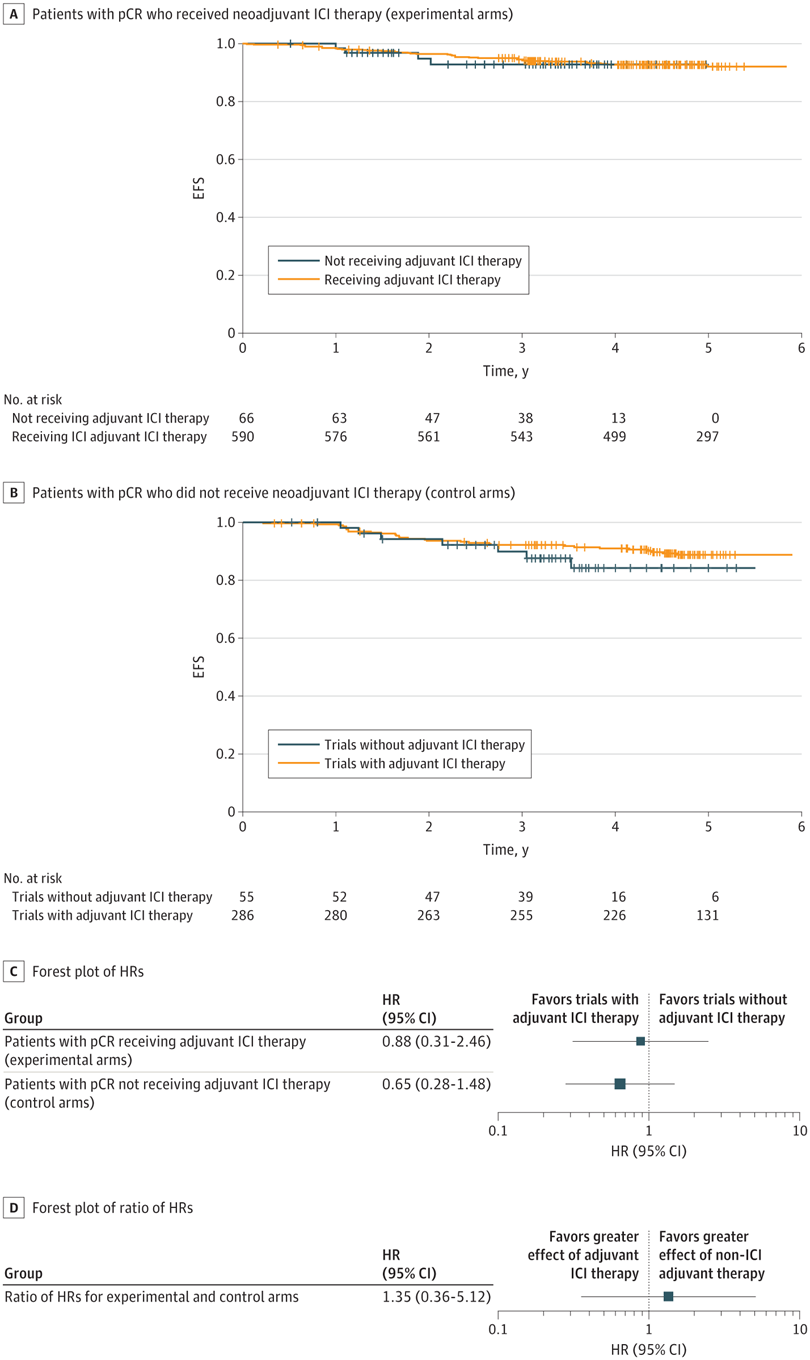
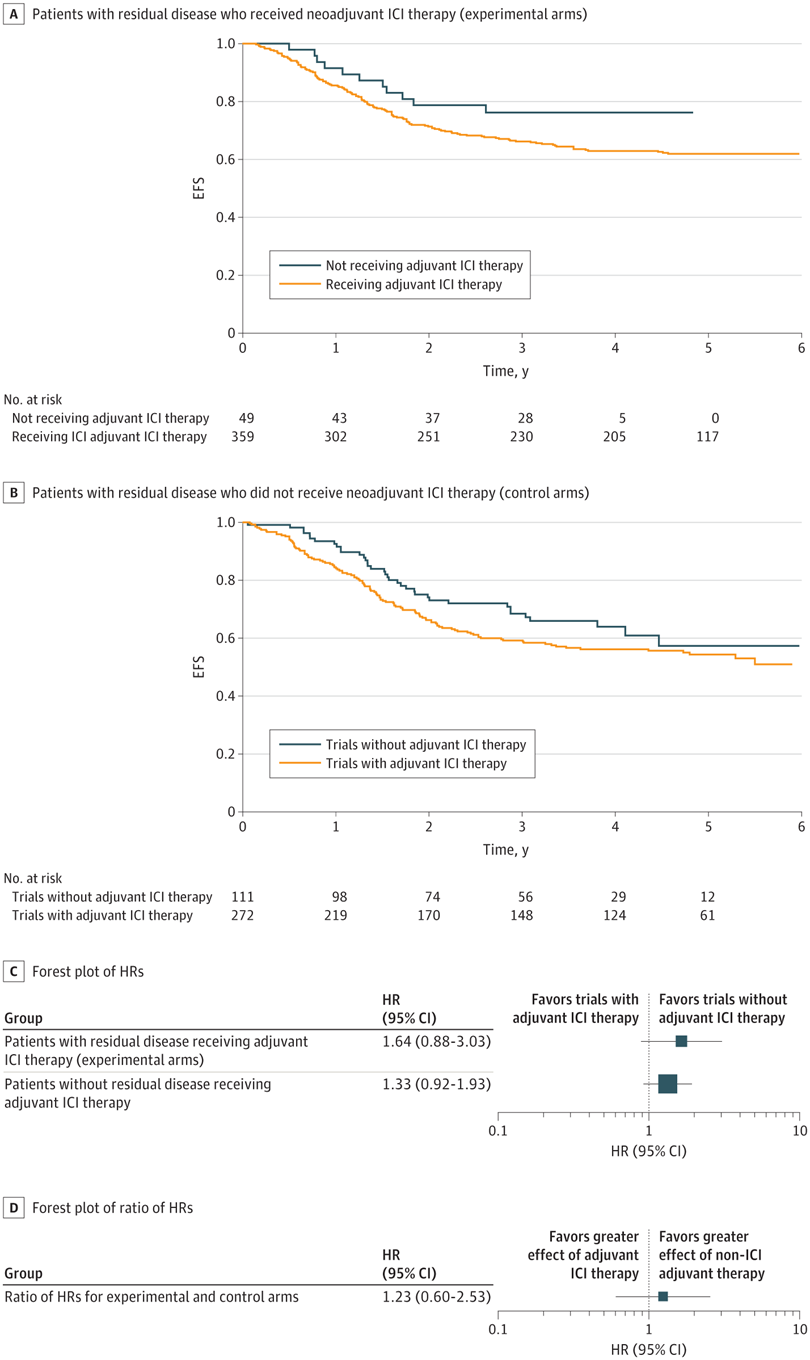
术前治疗期间,免疫检查点抑制剂≥3级免疫相关不良事件发生率为10.3% 。
因此,该研究结果表明,对于早期乳腺癌:术前化疗±免疫检查点抑制剂相比, 三阴性肿瘤、PD-L1阳性激素受体阳性HER2阴性肿瘤的病理完全缓解率显著较高,三阴性患者无事件生存率显著较高,且安全性可接受,HER2阳性肿瘤未见任何获益;术后化疗±免疫检查点抑制剂相比,未见任何获益。考虑到免疫检查点抑制剂相关经济成本和免疫检查点抑制所致毒性成本,进一步研究应该优先确定最有可能对术前化疗联合免疫检查点抑制剂获益的患者。
对此,美国哈佛大学医学院、布莱根医院和波士顿妇女医院、达纳法伯癌症研究院、达纳法伯布莱根癌症中心发表同期评论:术前免疫治疗——从试验到实践。
JAMA Oncol. 2024 Aug 29. IF: 22.5
Neoadjuvant Immune Checkpoint Inhibitors Plus Chemotherapy in Early Breast Cancer: A Systematic Review and Meta-Analysis.
Guillermo Villacampa, Victor Navarro, Alexios Matikas, Joana Mourato Ribeiro, Francesco Schettini, Pablo Tolosa, Olga Martínez-Sáez, Rodrigo Sánchez-Bayona, Juan M. Ferrero-Cafiero, Fernando Salvador, Andri Papakonstantinou, Aleix Prat, Mafalda Oliveira, Tomas Pascual.
SOLTI Cancer Research Group, Barcelona, Spain; Vall d'Hebron University Hospital, Vall d'Hebron Institute of Oncology, Barcelona, Spain; August Pi i Sunyer Biomedical Research Institute, Barcelona, Spain; Hospital Clinic of Barcelona, Barcelona, Spain; University of Barcelona, Barcelona, Spain; Reveal Genomics, Barcelona, Spain; Institute of Oncology-Quirón, Barcelona, Spain; Hospital Universitario 12 de Octubre, Madrid, Spain; Karolinska Institutet, Stockholm, Sweden; Karolinska University Hospital, Stockholm, Sweden; Université Paris-Saclay, Gustave Roussy, Villejuif, France.
This meta-analysis examines the efficacy outcomes and safety profile of immune checkpoint inhibitors in combination with adjuvant chemotherapy in patients with early breast cancer across molecular phenotypes .
QUESTION : What is the optimal approach for integrating immune checkpoint inhibitors (ICIs) in early-stage breast cancer?
FINDINGS : In this meta-analysis involving 5114 patients, neoadjuvant ICI therapy was associated with improved efficacy outcomes in early-stage triple-negative breast cancer and programmed cell death ligand 1-positive hormone receptor-positive/ERBB2-negative tumors with an acceptable safety profile. However, in triple-negative breast cancer, no benefit was observed with adjuvant ICI in patients with pathologic complete response or residual disease.
MEANING : These results favor neoadjuvant over adjuvant ICI therapy in the treatment of early-stage breast cancer.
IMPORTANCE : Recent studies have investigated the combination of immune checkpoint inhibitors (ICIs) with (neo)adjuvant chemotherapy in early-stage breast cancer. However, there is an ongoing debate about the optimal approach for integrating this strategy.
OBJECTIVES : To evaluate the association of neoadjuvant ICIs with pathologic complete response (pCR) across molecular phenotypes, to quantify the survival benefits of ICIs beyond pCR status, and to estimate the incidence of specific adverse events.
DATA SOURCES : The PubMed database was searched on December 10, 2023, to identify all potential eligible studies.
STUDY SELECTION : Randomized clinical trials (RCTs) that assessed (neo)adjuvant ICI plus chemotherapy in early breast cancer.
DATA EXTRACTION AND SYNTHESIS : Data from the eligible RCTs were extracted by 2 reviewers. An extracted individual patient data meta-analysis and a trial-level random-effect meta-analysis were performed.
MAIN OUTCOME(S) AND MEASURE(S) : Outcomes were pCR, event-free survival (EFS) in patients with and without pCR, and adverse events. Hazard ratios were estimated using stratified Cox proportional hazards regression models
RESULTS : Nine RCTs involving 5114 patients met the inclusion criteria (2097 triple-negative breast cancer [TNBC], 1924 hormone receptor-positive [HR+]/ERBB2-negative [ERBB2-], and 1115 ERBB2+ tumors). In TNBC, the addition of ICIs was associated with an improved pCR rate regardless of programmed cell death ligand 1 (PD-L1) status (absolute improvement, >10%). In HR+/ ERBB2- tumors, the administration of ICIs was associated with improved pCR only in the PD-L1-positive (PD-L1+) population (absolute improvement, +12.2%), whereas no benefit was observed in ERBB2+ tumors. In patients with TNBC achieving a pCR, the addition of ICIs was associated with improved EFS (hazard ratio, 0.65; 95% CI, 0.42-1.00), resulting in a 5-year EFS of 92.0% with ICIs compared with 88.0% without them. In patients with residual disease, ICIs also showed better EFS (hazard ratio, 0.77; 95% CI, 0.61-0.98), resulting in a 5-year EFS of 63.3% with ICIs and 56.1% without them. Adjuvant ICI did not show numerical improvement in patients with either pCR or residual disease (all hazard ratios >1). During the neoadjuvant treatment, the incidence of grade 3 or greater immune-related adverse events with ICI was 10.3%.
CONCLUSIONS AND RELEVANCE : These findings suggest that neoadjuvant ICI therapy improves efficacy outcomes in early-stage TNBC and PD-L1+ HR+/ERBB2- tumors with an acceptable safety profile; however, no benefit was observed with adjuvant ICI. Given the financial and toxicity costs associated with ICIs, future research should prioritize identifying patients most likely to benefit from the addition of ICIs to neoadjuvant chemotherapy.
DOI : 10.1001/jamaoncol.2024.3456
JAMA Oncol. 2024 Aug 29. IF: 22.5
Neoadjuvant Immunotherapy—From Trials to Practice.
Elizabeth A. Mittendorf, Sara M. Tolaney.
Brigham and Women's Hospital, Boston, Massachusetts; Dana-Farber Brigham Cancer Center, Boston, Massachusetts; Harvard Medical School, Boston, Massachusetts; Dana-Farber Cancer Institute, Boston, Massachusetts.
DOI : 10.1001/jamaoncol.2024.2924
略
本文仅供专业人士参看,文中内容仅代表美国医学会杂志社立场与观点,不代表肿瘤资讯平台意见,且肿瘤资讯并不承担任何连带责任。若有任何侵权问题,请联系删除。版权归文章作者所有,作者拥有所有法定权利。











 苏公网安备32059002004080号
苏公网安备32059002004080号


