
龚渭华 教授






摘要

近些年,不同的学者和机构提出了胃癌的各种分子分型方案。国际上两个主流的胃癌分子分型为TCGA分型和ACRG分型。在TCGA分型中,胃癌被分为4型:EB病毒阳性型、MSI型、基因组稳定型和染色体不稳定性型,其中基因组稳定型胃癌通常伴随CDH1和RHOA基因突变,并且细胞黏附通路表达升高[2]。在ACRG分型中,胃癌也分为4型:MSI型、微卫星稳定/上皮-间充质转化型(MSS/EMT)、微卫星稳定/TP53+型(MSS/TP53+)和微卫星稳定/TP53-型(MSS/TP53-)[3]。与TCGA分型相比,ACRG分型与患者预后更为相关。在ACRG分型中,MSI型胃癌预后最好,MSS型胃癌预后差,其中MSS/EMT型胃癌预后最差。另外,ACRG胃癌分子分型与胃癌患者预后的关系也在其他数据集(TCGA胃癌数据集、GSE15459、GSE26253和SMC-2数据集)中得到了很好验证。

在临床中,通常通过免疫组化的方法检测MMR系统中的MLH1、MSH2,MSH6和PMS2这4个蛋白表达情况,只要其中1个蛋白不表达,即为dMMR状态。dMMR状态和MSI-H状态的符合率在90%以上。检测微卫星状态也可以通过多重荧光PCR的方法检测MSI的标志物。2024版中国临床肿瘤学会胃癌指南就推荐检测美国国家癌症研究所推荐的5个微卫星位点,包括2个单核苷酸位点和3个双核苷酸位点。当这5个核苷酸位点都稳定,即为MSS,当其中1个不稳定,即为低度微卫星不稳定(microsatellite instability low,MSI-L),若有2个或以上不稳定,即为MSI-H。随着更多标志物被发现可用于微卫星检测,MSI-H被重新定义为至少有30%~40%不稳定标志物者,而MSI-L定义为低于30%~40%不稳定标志物者。

1.手术:对于cT1aN0M0的早期胃癌,可行内镜下黏膜切除术(endoscopic mucosal resection,EMR)或内镜黏膜下剥离术(endoscopic submucosal dissection,ESD)。EMR和ESD最早的适应证为直径<2 cm且分化良好不伴溃疡形成的黏膜内癌。ESD的适应证在不断扩展,随着JCOG0607研究和JCOG1009/1010研究结果的发表,日本将直径>2 cm的不伴溃疡分化型黏膜内癌、直径<3 cm的伴溃疡分化型黏膜内癌和直径<2 cm的不伴溃疡未分化型黏膜内癌也纳入ESD的适应证[6,7]。除了ⅣB期胃癌,只要无不可手术切除因素都满足手术适应证。手术的方式包括传统的开放手术、腹腔镜手术和机器人手术。对于早期胃癌,KLASS-01研究、JCOG0912研究和CLASS02研究的结果表明腹腔镜行远端胃切除术和全胃切除术与传统开放手术相比具有同样的安全性[8,9,10]。对于局部进展期胃癌,D2组淋巴结清扫已基本达成共识。CLASS-01研究是中国发起的首个研究腹腔镜行远端胃切除术和淋巴结清扫对比开放手术治疗局部进展期胃癌的研究。该研究发现两组患者术后3年和5年生存率差异无统计学意义,而腹腔镜组术后恢复更快,并发症更少[11]。KLASS-02研究和JLSSG0901研究同样发现腹腔镜下行远端胃切除术和淋巴结清扫具有和开放手术一样的安全性,并且3年和5年无复发生存率差异无统计学意义[12,13]。达芬奇机器人手术的优点包括有着更高的放大倍数、术者操作更灵活和更微创,近些年来越来越多用在胃癌手术上。多项研究发现达芬奇机器人手术与腹腔镜手术相比对胃癌患者长期生存的影响无明显差异,并且机器人手术在术中出血量和淋巴结清扫上存在优势[14,15,16]。目前尚缺乏关于达芬奇机器人手术对比腹腔镜手术治疗胃癌的高质量前瞻性研究结果。由于达芬奇机器人手术费用昂贵,目前难以普及。
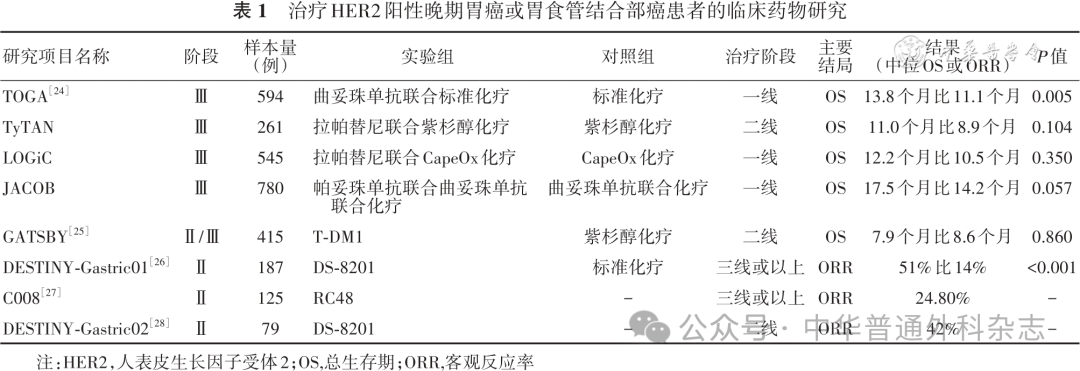

MSS型胃癌是胃癌最常见的类型。对于MSS型胃癌患者,早期治疗主要以手术为主,局部进展期治疗通常需要围手术期化疗加上手术。晚期MSS型胃癌患者需要接受以化疗为主的系统治疗,可根据HER2表达情况选择曲妥珠单抗靶向治疗。因晚期MSS型胃癌患者对PD1/PD-L1抑制剂整体反应率低,需综合多种因素(EB病毒感染、PD-L1表达水平)个性化选择免疫治疗药物,可考虑使用其他靶点和双靶点免疫检查点抑制剂。
[1] Furukawa K , Hatakeyama K , Terashima M ,et al. Molecular features and prognostic factors of locally advanced microsatellite instability-high gastric cancer[J]. Gastric Cancer, 2024,27(4):760-771. DOI: 10.1007/s10120-024-01506-5 .
[2] Comprehensive molecular characterization of gastric adenocarcinoma[J]. Nature, 2014,513(7517):202-209. DOI: 10.1038/nature13480 .
[3] Cristescu R , Lee J , Nebozhyn M ,et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes[J]. Nat Med, 2015,21(5):449-456. DOI: 10.1038/nm.3850 .
[4] Chen Y , Jia K , Sun Y ,et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment[J]. Nat Commun, 2022,13(1):4851. DOI: 10.1038/s41467-022-32570-z .
[5] Bradford KC , Wilkins H , Hao P ,et al. Dynamic human MutSα-MutLα complexes compact mismatched DNA[J]. Proc Natl Acad Sci USA, 2020,117(28):16302-16312. DOI: 10.1073/pnas.1918519117 .
[6] Hasuike N , Ono H , Boku N ,et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607)[J]. Gastric Cancer, 2018,21(1):114-123. DOI: 10.1007/s10120-017-0704-y .
[7] Takizawa K , Ono H , Hasuike N ,et al. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010)[J]. Gastric Cancer, 2021,24(2):479-491. DOI: 10.1007/s10120-020-01134-9 .
[8] Kim HH , Han SU , Kim MC ,et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage Ⅰ gastric cancer: the KLASS-01 randomized clinical trial[J]. JAMA Oncol, 2019,5(4):506-513. DOI: 10.1001/jamaoncol.2018.6727 .
[9] Katai H , Mizusawa J , Katayama H ,et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage ⅠA or ⅠB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial[J]. Lancet Gastroenterol Hepatol, 2020,5(2):142-151. DOI: 10.1016/s2468-1253(19)30332-2 .
[10] Liu F , Huang C , Xu Z ,et al. Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage Ⅰ gastric cancer: the CLASS02 multicenter randomized clinical trial[J]. JAMA Oncol, 2020,6(10):1590-1597. DOI: 10.1001/jamaoncol.2020.3152 .
[11] Huang C , Liu H , Hu Y ,et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial[J]. JAMA Surg, 2022,157(1):9-17. DOI: 10.1001/jamasurg.2021.5104 .
[12] Hyung WJ , Yang HK , Park YK ,et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial[J]. J Clin Oncol, 2020,38(28):3304-3313. DOI: 10.1200/jco.20.01210 .
[13] Etoh T , Ohyama T , Sakuramoto S ,et al. Five-year survival outcomes of laparoscopy-assisted vs open distal gastrectomy for advanced gastric cancer: the JLSSG0901 randomized clinical trial[J]. JAMA Surg, 2023,158(5):445-454. DOI: 10.1001/jamasurg.2023.0096 .
[14] Shin HJ , Son SY , Wang B ,et al. Long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer: a propensity score-weighted analysis of 2 084 consecutive patients[J]. Ann Surg, 2021,274(1):128-137. DOI: 10.1097/sla.0000000000003845 .
[15] De Jongh C , Cianchi F , Kinoshita T ,et al. Surgical techniques and related perioperative outcomes after robot-assisted minimally invasive gastrectomy (RAMIG): results from the prospective multicenter international ugira gastric registry[J]. Ann Surg, 2024,280(1):98-107. DOI: 10.1097/sla.0000000000006147 .
[16] Li ZY , Zhou YB , Li TY ,et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: a multicenter cohort study of 5 402 patients in China[J]. Ann Surg, 2023,277(1):e87-e95. DOI: 10.1097/sla.0000000000005046 .
[17] Cunningham D , Allum WH , Stenning SP ,et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer[J]. N Engl J Med, 2006,355(1):11-20. DOI: 10.1056/NEJMoa055531 .
[18] Al-Batran SE , Homann N , Pauligk C ,et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial[J]. Lancet, 2019,393(10184):1948-1957. DOI: 10.1016/s0140-6736(18)32557-1 .
[19] Zhang X , Liang H , Li Z ,et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial [J]. Lancet Oncol, 2021,22(8):1081-1092. DOI: 10.1016/s1470-2045(21)00297-7 .
[20] Kang YK , Yook JH , Park YK ,et al. PRODIGY: a phase Ⅲ study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer[J]. J Clin Oncol, 2021,39(26):2903-2913. DOI: 10.1200/jco.20.02914 .
[21] Sakuramoto S , Sasako M , Yamaguchi T ,et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine[J]. N Engl J Med, 2007,357(18):1810-1820. DOI: 10.1056/NEJMoa072252 .
[22] Bang YJ , Kim YW , Yang HK ,et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial[J]. Lancet, 2012,379(9813):315-321. DOI: 10.1016/s0140-6736(11)61873-4 .
[23] Zhao F , Li E , Shen G ,et al. Correlation between mismatch repair and survival of patients with gastric cancer after 5-FU-based adjuvant chemotherapy [J]. J Gastroenterol, 2023,58(7):622-632. DOI: 10.1007/s00535-023-01990-z .
[24] Bang YJ , Van Cutsem E , Feyereislova A ,et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2010,376(9742):687-697. DOI: 10.1016/s0140-6736(10)61121-x .
[25] Thuss-Patience PC , Shah MA , Ohtsu A ,et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal ju nction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study [J]. Lancet Oncol, 2017,18(5):640-653. DOI: 10.1016/s1470-2045(17)30111-0 .
[26] Shitara K , Bang YJ , Iwasa S ,et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer[J]. N Engl J Med, 2020,382(25):2419-2430. DOI: 10.1056/NEJMoa2004413 .
[27] Peng Z , Liu T , Wei J ,et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase Ⅱ study[J]. Cancer Commun, 2021,41(11):1173-1182. DOI: 10.1002/cac2.12214 .
[28] Van Cutsem E , Di Bartolomeo M , Smyth E ,et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study[J]. Lancet Oncol, 2023,24(7):744-756. DOI: 10.1016/s1470-2045(23)00215-2 .
[29] Kubota Y , Kawazoe A , Mishima S ,et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal jun ction cancer [J]. ESMO Open, 2023,8(1):100762. DOI: 10.1016/j.esmoop.2022.100762 .
[30] Shitara K , Lordick F , Bang Y J ,et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial[J]. Lancet, 2023,401(10389):1655-1668. DOI: 10.1016/s0140-6736(23)00620-7 .
[31] Shah MA , Shitara K , Ajani JA ,et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW tria l [J]. Nat Med, 2023,29(8):2133-2141. DOI: 10.1038/s41591-023-02465-7 .
[32] Wainberg ZA , Enzinger PC , Kang YK ,et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study[J]. Lancet Oncol, 2022,23(11):1430-1440. DOI: 10.1016/s1470-2045(22)00603-9 .
[33] Shitara K , Van Cutsem E , Bang Y J ,et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial[J]. JAMA Oncol, 2020,6(10):1571-1580. DOI: 10.1001/jamaoncol.2020.3370 .
[34] Janjigian YY , Shitara K , Moehler M ,et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial[J]. Lancet, 2021,398(10294):27-40. DOI: 10.1016/s0140-6736(21)00797-2 .
[35] Rha SY , Oh DY , Yañez P ,et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial[J]. Lancet Oncol, 2023,24(11):1181-1195. DOI: 10.1016/s1470-2045(23)00515-6 .
[36] Xu J , Jiang H , Pan Y ,et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial[J]. Jama, 2023,330(21):2064-2074. DOI: 10.1001/jama.2023.19918 .
[37] Janjigian YY , Bendell J , Calvo E ,et al. Checkmate-032 study: efficacy and safety of Nivolumab and Nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer[J]. J Clin Oncol, 2018,36(28):2836-2844. DOI: 10.1200/jco.2017.76.6212 .
[38] Gao X , Ji K , Jia Y ,et al. Cadonilimab with chemotherapy in HER2-negative gastric or gastroesophageal junction adenocarcinoma: the phase 1b/2 COMPASSION-04 trial[J]. Nat Med, 2024,30(7):1943-1951. DOI: 10.1038/s41591-024-03007-5 .
[39] Shitara K , Rha SY , Wyrwicz LS ,et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study[J]. Lancet Oncol, 2024,25(2):212-224. DOI: 10.1016/s1470-2045(23)00541-7 .
[40] Kang YK , Terashima M , Kim YW ,et al. Adjuvant nivolumab plus chemotherapy versus placebo plus chemotherapy for stage III gastric or gastro-oesophageal junction cancer after gastrectomy with D2 or more extensive lymph-node dissection (ATTRACTION-5): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2024,9(8):705-717. DOI: 10.1016/s2468-1253(24)00156-0 .











 苏公网安备32059002004080号
苏公网安备32059002004080号


