目前,左心室射血分数被指南推荐用于乳腺癌化疗患者心功能监测,预测化疗所致心力衰竭,尤其 左心室收缩功能下降 。不过,近年来不少研究指出,由于左心室射血分数对于预测室性心律失常以及心原性猝死事件的敏感性和特异性都不理想,故有必要寻找更好的监测指标,以便及时启动心脏保护治疗。 整体纵向应变是评价左心室整体纵向收缩功能的重要指标,无角度依赖性,与左心室射血分数相比,可以检测到心肌机械运动细微变化,更早识别心肌收缩功能受损。
2024年9月1日,欧洲心脏病学会官方期刊《欧洲心脏杂志》在线发表澳大利亚贝克心脏和糖尿病研究所、塔斯马尼亚大学孟席斯医学研究所、彼得麦卡伦癌症中心、皇家阿德莱德医院、艾德华心脏病医院、昆士兰大学医学院、皇家布里斯班医院和妇女医院、尼皮恩医院、纽卡斯尔大学心脏肿瘤学中心、亨特医学研究所、加略山圣母医院、亨特新英格兰健康中心、亚历山德拉公主医院、利物浦医院、弗林德斯大学、悉尼大学和新南威尔士大学韦斯特米德临床医学院、加拿大多伦多综合医院彼得蒙克心脏中心的化疗期间磁共振成像应变监测以改善心血管结局(SUCCOUR-MRI)研究报告,对整体纵向应变恶化但是左心室射血分数正常患者是否启动心脏保护治疗的心血管结局进行了比较。
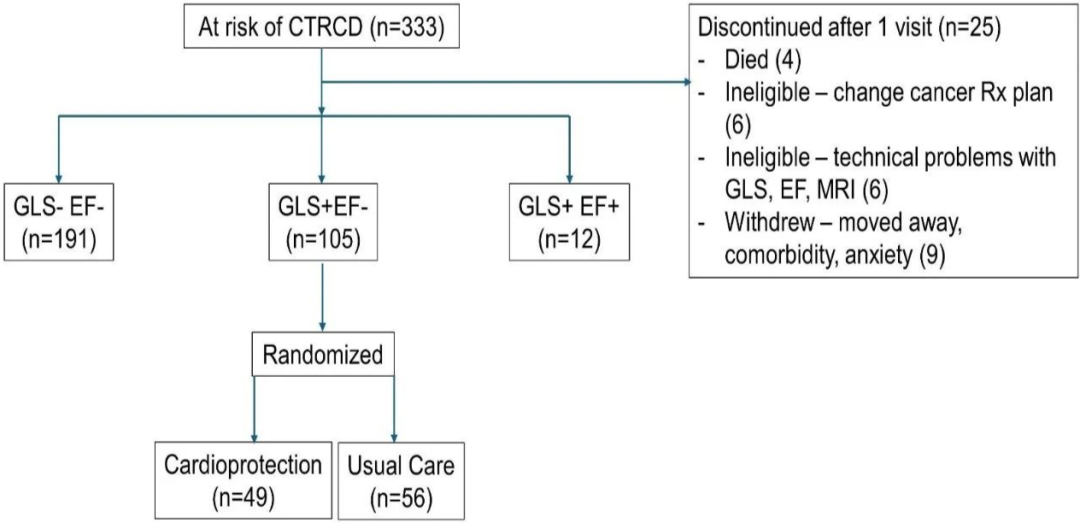
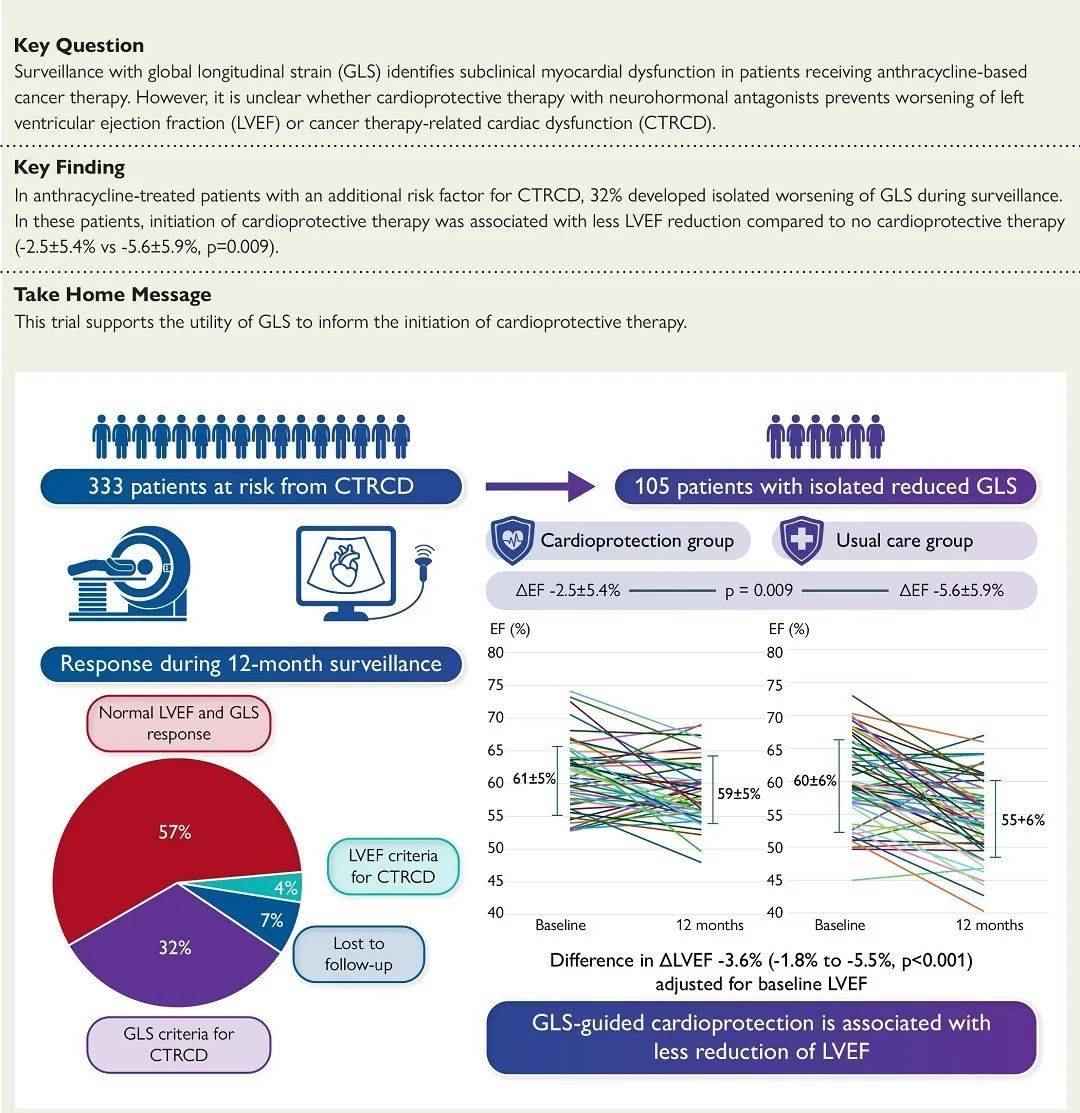
该多中心前瞻随机对照试验于2018年至2023年8月从澳大利亚全国14家医院355例接受蒽环类治疗并且治疗前左心室射血分数正常患者中,筛选出至少有一项其他癌症治疗相关心功能障碍风险因素、能够进行磁共振成像整体纵向应变和三维超声心动图检查的333例患者(年龄59±13岁,79%为女性)进行12个月随访。
总计105例患者(年龄59±13岁,75%为女性,69%为乳腺癌)发生癌症治疗相关整体纵向应变心功能障碍(整体纵向应变相对减少>12%,左心室射血分数未变化)被随机分为两组:
其中49例给予心脏保护 其余56例给予常规治疗
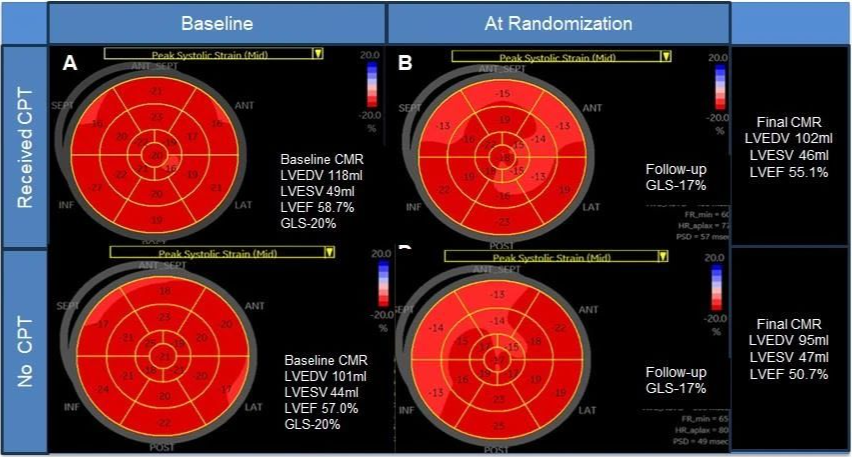
主要终点为磁共振成像左心室射血分数12个月变化;次要终点为磁共振成像左心室射血分数定义的癌症治疗相关心功能障碍。
结果,随访期间2例患者死亡、2例患者发生心力衰竭。大多数患者(62%)在3个月时被随机分组。
血管紧张素抑制或阻断剂和β受体阻滞剂的中位剂量分别为各自目标剂量的75%和50%;21例(43%)患者出现归因于心脏保护的副作用。
心脏保护组 与常规治疗组的患者相比:
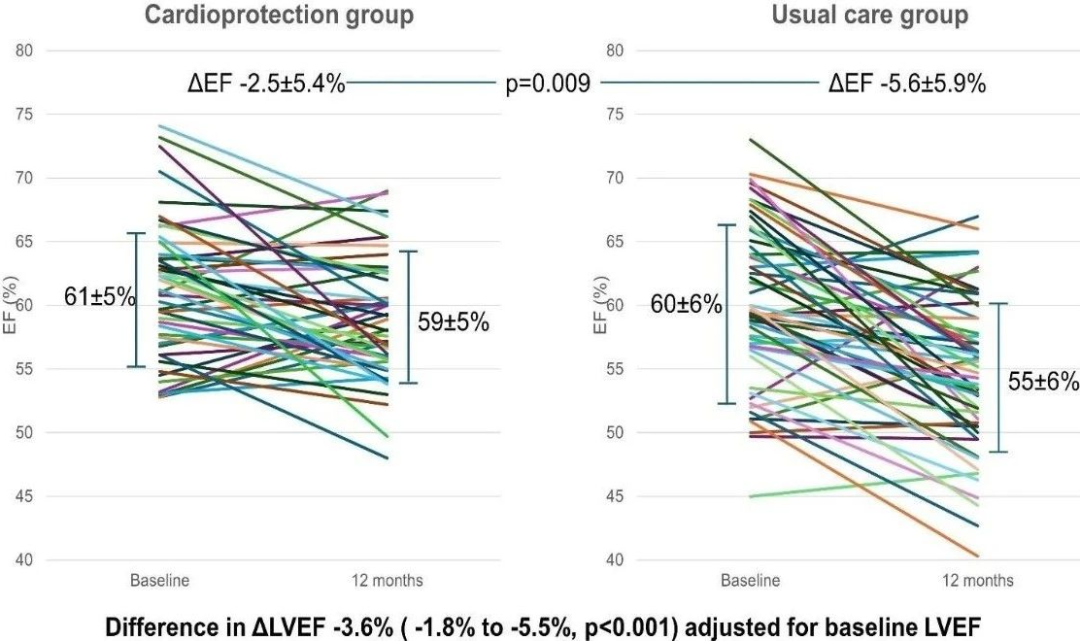
左心室射血分数降低显著较少:-2.5±5.4%比-5.6±5.9%(P=0.009) 随访左心室射血分数显著较高:59±5%比55±6%(P<0.0001) 对治疗前左心室射血分数进行校正后左心室射血分数变化平均相差:-3.6%(95%置信区间:-1.8%~-5.5%,P<0.001) 12个月癌症治疗相关左心室射血分数心功能障碍发生率显著较低:2.0%比10.7%(P=0.075)
心脏保护组患者随机分组后3个月内整体纵向应变有所改善,常规治疗组患者几乎未变化。
因此,该研究结果表明,对于蒽环类化疗后仅仅出现整体纵向应变减少而左心室射血分数正常的患者,心脏保护与常规治疗相比,12个月磁共振成像左心室射血分数降低显著较少。
Eur Heart J. 2024 Sep 1. IF: 37.6
Strain surveillance during chemotherapy to improve cardiovascular outcomes: the SUCCOUR-MRI trial.
Marwick TH, Dewar E, Nolan M, Shirazi M, Dias P, Wright L, Fitzgerald B, Kearney L, Srivastava P, Atherton J, Negishi K, Sverdlov AL, Wahi S, Otton J, Selvanayagam J, Thomas L, Thavendiranathan P; SUCCOUR-MRI investigators.
Baker Heart and Diabetes Institute, Melbourne, Australia; Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; Peter MacCallum Cancer Centre, Melbourne, Australia; Royal Adelaide Hospital, Adelaide, Australia; Advara Heart Care, Murdoch, Australia; University of Queensland Faculty of Medicine, Royal Brisbane and Women's Hospital, Brisbane, Australia; Nepean Hospital, Kingswood, Australia; Newcastle Centre of Excellence in Cardio-Oncology, The University of Newcastle, Hunter Medical Research Institute, Calvary Mater Newcastle, Hunter New England Health, Newcastle, Australia; Princess Alexandra Hospital, Brisbane, Australia; Liverpool Hospital, Liverpool, Australia; Flinders University, Adelaide, Australia; Westmead Clinical school, University of Sydney and University of New South Wales, Sydney, Australia; Peter Munk Cardiac Centre, Toronto General Hospital, Toronto, Canada.
BACKGROUND AND AIMS : The detection of cancer therapy-related cardiac dysfunction (CTRCD) by reduction of left ventricular ejection fraction (LVEF) during chemotherapy usually triggers the initiation of cardioprotective therapy. This study addressed whether the same approach should be applied to patients with worsening of global longitudinal strain (GLS) without attaining thresholds of LVEF.
METHODS : Strain sUrveillance during Chemotherapy for improving Cardiovascular Outcomes (SUCCOUR-MRI) was a prospective multicentre randomized controlled trial involving 14 sites. Of 355 patients receiving anthracyclines with normal baseline LVEF, 333 patients (age 59±13 years, 79% women) with at least one other CTRCD risk factor, able to undergo magnetic resonance imaging (MRI), GLS and 3D echocardiography were tracked over 12 months. A total of 105 patients (age 59±13 years, 75% women, 69% breast cancer) developing GLS-CTRCD (>12% relative reduction of GLS without a change in LVEF) between cardioprotection with neurohormonal antagonists versus usual care were randomized. The primary endpoint was 12-month change in MRI-LVEF; the secondary endpoint was MRI LVEF-defined CTRCD.
RESULTS : During follow-up, 2 patients died and 2 developed heart failure. Most patients were randomized at 3 months (62%). Median doses of angiotensin inhibition/blockade and beta-blockade were 75% and 50% of respective targets; 21 (43%) had side-effects attributed to cardioprotection. Due to a smaller LVEF change from baseline with cardioprotection than usual care (-2.5±5.4% vs -5.6±5.9%, p=0.009), follow-up LVEF was higher after cardioprotection (59±5% vs 55±6%, p<0.0001). After adjustment for baseline LVEF, the mean (95% confidence interval) difference in the change in LVEF between the two groups was -3.6% (-1.8% to -5.5%, p<0.001). After cardioprotection, 1/49 patients developed 12-month LVEF-CTRCD, compared to 6/56 in usual care (p=0.075). GLS improved at 3 months post-randomization in the cardioprotection group, with little change with usual care.
CONCLUSIONS : In patients with isolated GLS reduction after anthracyclines, cardioprotection is associated with better preservation of 12-month MRI-LVEF compared with usual care.
KEYWORDS : cancer therapy-related cardiac dysfunction; global longitudinal strain; left ventricular ejection fraction.
PMID : 39217601
DOI : 10.1093/eurheartj/ehae574
略











 苏公网安备32059002004080号
苏公网安备32059002004080号


