
本文刊登于《中国实用妇科与产科杂志》 2024,40( 7 ): 729-736
DOI:10.19538/j.fk2024070113
【引用本文】中国老年保健协会妇科肿瘤专业委员会.老年卵巢癌患者围手术期管理专家共识(2024年版)[J].中国实用妇科与产科杂志, 2024,40( 7 ): 729-736 .
作者: 中国老年保健协会妇科肿瘤专业委员会
基金项目: 国家自然科学基金(82372126,82072078);中大医院-盱眙县人民医院联合基金(zdyyxy07)
通信作者: 沈杨,东南大学附属中大医院,江苏 南京 210009,电子信箱:shenyang@seu.edu.cn
指导专家: 孔北华(山东大学齐鲁医院)
执笔专家(按姓氏汉语拼音排序): 陈小军(复旦大学附属肿瘤医院);陈小祥(江苏肿瘤医院);申震(中国科学技术大学附属第一医院);沈杨(东南大学附属中大医院);孙丽(青岛市中心医院);武欣(复旦大学附属妇产科医院);殷霞(上海交通大学医学院附属仁济医院);周颖(中国科学技术大学附属第一医院);朱滔(浙江省肿瘤医院);邹冬玲(重庆大学附属肿瘤医院)
参与共识讨论与制定专家(按姓氏汉语拼音排序): 陈曦(浙江省肿瘤医院);成磊[山东大学齐鲁医院(青岛)];丁波(东南大学附属中大医院);郝晓园(徐州矿务集团总医院);何心勤(福建医科大学附属第一医院);侯文杰(苏州大学附属独墅湖医院);黄海伟(张家港市第一人民医院);金平(深圳市妇幼保健院);孔为民(北京妇产医院);雷玲(安顺市人民医院);李大可(南京市妇幼保健院);李敏(中国科学技术大学附属第一医院);李妍(宁夏医科大学总医院);凌开建(陆军军医大学第一附属医院);刘畅(兰州大学第一附属医院);卢淮武(中山大学孙逸仙纪念医院);邵株燕(浙江省肿瘤医院);孙冬梅(盱眙县人民医院);唐雪栋(嘉兴市妇幼保健院);王宁(大连医科大学第二附属医院);邢艳霞(青海省第五人民医院);徐浩(东南大学附属中大医院);徐敬云(东南大学附属中大医院);徐臻(郑州大学第二附属医院);许波群(南京医科大学第二附属医院);杨立(郑州大学第三附属医院);杨萍(石河子大学医学院第一附属医院);张春花(淮安市妇幼保健院);张天骄(中国科学技术大学附属第一医院);张玮(复旦大学附属肿瘤医院);张阳(连云港市第一人民医院);张英丽(浙江省肿瘤医院);周金华(苏州大学第一附属医院);周秀芬(六安市人民医院);朱靖(中国科学技术大学附属第一医院);左欣(宜兴市人民医院)
写作秘书: 张天骄(中国科学技术大学附属第一医院);丁波(东南大学附属中大医院)
1.1 多学科诊疗 因卵巢癌治疗存在复杂性和长期性,多学科诊疗(multi-disciplinary treatment,MDT)模式显得尤为重要[7-9]。术前应根据患者一般情况、肿瘤扩散程度、手术可能涉及的范围,进行包括但不限于妇科肿瘤科、麻醉科、外科、影像科、病理科、营养科、重症监护科、内科等的多学科诊疗及讨论。讨论内容包括明确诊断,制定合适的治疗模式和最优的诊疗方案,手术途径方式、风险及可行性,术中麻醉注意事项,术后特殊辅助治疗的选择及安全性管理,诊疗过程可能出现的相关并发症等。
1.2 综合评估 老年综合评估(comprehensive geriatric assessment,CGA)是通过多学科评估方法对老年患者的身体精神状态、心理健康状态、社会经济状况、营养状况、多重用药等多个维度健康状态进行的测量和评估,并据此制订以维持和改善老年患者健康及功能状态为目的的治疗计划,最大限度地提高老年患者的生活质量[10]。CGA评估体系是老年肿瘤患者评估的核心,对治疗具有指导意义。NCCN老年肿瘤小组建议对所有患有癌症的老年患者使用CGA进行治疗前评估,且CGA评估体系应当贯穿于治疗的始终。多种方法均可用于CGA的评估,包括G8、改良G8、VES-13、改良CGA[11]等均可作为参考。

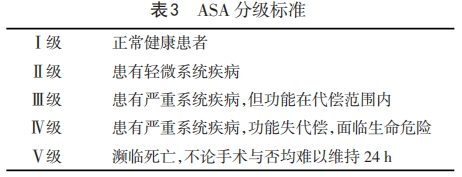
1.3 麻醉评估 麻醉前评估应基于美国麻醉医师协会(American Society of Anesthesiology,ASA)分级标准(表3),对患者身体条件与合并症进行总的术前评估。在老年患者的评估中,强调从低维度转向多维度评估、筛查、干预与随访的转变,在多学科的协助下为患者制定个体化检测、管理方案。对麻醉与手术相关死亡率的研究发现,整体人群的总死亡率为1.2%,其中60~69岁组为2.2%,70~79岁组为2.9%,80岁及以上组为5.8%,90岁及以上组为8.4%[24]。
1.4 内科系统评估
1.4.1 心血管系统 欧洲心血管病协会 (European Society of Cardiology,ESC)[26]、美国心脏病学会 (American College of Cardiology,ACC)及美国心脏协会(American Heart Association,AHA)[27]颁布了术前心脏评估指南,用来指导老年患者术前的心脏评估及干预。
1.4.2 呼吸系统 肺部评估需做好以下几方面工作:详细的病史采集和体格检查,在术前应明确患者的活动耐力情况和肺部疾病情况(肺功能、血气分析等);术前控制慢性阻塞性肺疾病(chronic obstructive pulmonary disease,COPD)、哮喘等疾病至最佳状态,必要时加用抗生素,哮喘患者应慎用β受体阻滞剂,以免诱发和加重哮喘;肺部、膈肌有无病灶转移,对肺功能有无影响,有无胸腔积液,术前是否需要胸腔闭式引流等治疗;戒烟;术前加强呼吸肌、咳嗽训练;解释术后如何做好肺功能恢复锻炼并有效镇痛。若肺功能第一秒用力呼气容积(FEV1)<600 mL,第一秒用力呼气容积占用力占肺活量之比值(FEV1%)<50%,动脉血氧分压(PaO2)<60 mmHg,则术后发生坠积性肺炎或咳痰困难的可能性大。
1.4.3 内分泌系统 主要关注指标为患者血糖水平,糖尿病患者口服降糖药物应注意进食情况,应根据患者进食量的变化随时调整药物剂量,以避免低血糖及高血糖的发生,同时维持水电解质平衡。建议将老年卵巢癌患者围手术期血糖控制在7.8~10.0 mmol/L。老年糖尿病的患者围手术期的血糖主要控制策略应为严格避免低血糖,尽量避免出现血糖≤3.9 mmol/L。对于年龄≥75岁、频繁发作低血糖、合并严重心脑血管疾病的患者,血糖目标上限适当放宽至12.0 mmol/L,最高不超过13.9 mmol/L。当血糖超过上述范围时,可考虑胰岛素治疗,并监测血糖,警惕低血糖的发生[28-29]。
1.4.4 卒中风险 颈动脉彩超与经颅彩色多普勒超声检测技术联合应用,可以及时准确地观察缺血性脑血管产生的颅内、外血流动力学变化,提高颅内外脑血管疾病的检出率和诊断正确率[30]。如果是轻度狭窄,一般不用特殊干预,可通过药物改善。如果是中重度狭窄,要进一步做数字减影血管造影(DSA)检查明确,并在术前给予相应治疗。同时根据评估结果,选择有效的预防性措施,如加强术中血压的监测、维持血压在基线水平以上并选择更安全的麻醉和手术方式。
1.4.5 血液系统 术前需识别及纠正贫血。若有贫血,口服铁剂应从小剂量开始,并且服用时间最好在两餐之间。严重贫血患者可考虑术前纠正贫血。指南推荐,对于非心脏手术的术前输血,血红蛋白(Hb)<70g/L有红细胞输注指征[31-32]。
1.5 静脉血栓栓塞性疾病的综合管理 静脉血栓栓塞症(venous thromboembolism,VTE)包括深静脉血栓形成(deep venousthrimbosis,DVT)和肺栓塞(pulmonaryembolism,PE)。卵巢癌VTE的高危因素包括肿瘤因素(手术病理分期为Ⅲ~Ⅳ期、合并腹水、组织病理学类型为透明细胞癌、高肿瘤负荷等)、患者因素[高龄、合并感染、肺及肾脏等并发症、术后制动、体重指数(BMI)>30和既往VTE病史等]及治疗因素(手术、化疗、放疗、激素治疗、抗血管生成治疗、应用促红细胞生成素、输血和留置静脉通路等)[33-35]。
2.1 术中一般管理 手术时体位为人字位(剪刀型体位);术中应严密监测患者生命体征变化,保证输液管路的通畅;如术中可能需要快速输注液体和血液制品,则应术前放置深静脉导管;预计可能大量失血时,需及时另外建立血管通路以便输血[40,44]。
2.2 术中麻醉和液体管理 患者个体差异大,麻醉的选择应综合考虑手术类型、时长、需求、患者状态等因素,可采用全身麻醉、区域阻滞或两者联合;术中严密监测患者生命体征和循环系统变化,根据有创动脉血压状态调整麻醉策略。血管活性药物的合理使用是优化患者全身血管张力的关键,应结合患者术前的基础情况,维持相对高水平血压(基础血压的90%~110%),维持心率在术前基线水平的80%~120%,避免循环的剧烈波动。
3.1 术后镇痛 术后疼痛可引起患者全身应激反应,严重影响患者术后舒适度和术后恢复。推荐建立联合麻醉科、妇瘤科和护理学科等多学科疼痛管理团队,使用全身和区域镇痛技术等多模式镇痛策略,以控制术后疼痛,使患者早期活动、早期恢复肠道营养以及减轻围手术期应激反应[54]。术后镇痛避免单一全身性阿片类药物的使用,推荐联合对乙酰氨基酚等非甾体类抗炎药行多模式镇痛。开腹行肿瘤细胞减灭术,因手术范围广泛,患者术后的疼痛严重,区域阻滞(如腹横肌平面神经阻滞)联合自控式镇痛泵是较为理想的镇痛方式[55]。
3.2 术后营养支持方案 对老年患者而言,传统的术后“开放性”或“限制性”液体治疗均存在各自缺陷。“开放性”的液体治疗可导致老年患者容量过负荷,增加发生肺水肿及心力衰竭的风险;而“限制性”液体治疗可导致有效循环容量不足、低血压及组织低灌注,不利于患者术后恢复。因此,老年患者的液体治疗应根据机体实时状态进行个体化调整,以优化血流动力学并保证组织灌注,而非简单的“开放性”或“限制性”液体治疗[56-57]。
3.3 术后早期下床活动和肠道功能恢复 推荐术后需采取多种措施促进患者肠道功能的恢复,具体措施包括:多模式镇痛、减少阿片类药物用量,控制液体入量,不留置鼻胃管,咀嚼口香糖、早期进食和下床活动。研究显示,早期下床活动与早期胃肠道的功能恢复、降低血栓栓塞等有关,因此推荐术后24 h内开始下床活动[62],可考虑使用乳果糖及中医中药等缓泻剂[40]。
3.4 术后出院和随访 出院标准应结合病情及术后恢复情况,做到个体化。基本的出院标准包括:恢复半流质饮食;停止静脉补液;口服镇痛药物可控制疼痛;创口愈合良好,无感染迹象;肠道正常排气排便;器官功能状态良好,可独立活动(能够在没有帮助的情况下起床并至少短距离行走)[40]。出院后24~48 h内应常规对患者进行电话随访,包括出院后指导、疼痛评估、创口护理、出院后并发症的监测;术后7~14 d患者应至门诊回访,回访内容包括创口拆线、查询病理检查结果、关注出院后并发症、制定后续治疗计划[40]。
3.5 术后化疗 目前术后到化疗开始的最佳开始时间仍有待确定,推荐术后应尽快开始化疗,推荐时间不迟于术后30~42 d[63-64]。术后化疗应结合患者的一般状态、有无基础疾病、术中及术后状况、体力状况评分综合决定。如一般状态差、有合并症、年龄≥70岁,其化疗毒性和治疗中断率增加,生存期缩短[65]。关于化疗方案的选择,本共识参照以及结合NCCN指南的建议执行[66]。强调对于年龄≥70岁或有并发症的患者,推荐剂量为:(1) 紫杉醇135 mg/m2+卡铂AUC 5,3周为1个周期,共3~6个周期。(2)小剂量周疗方案:紫杉醇60 mg/m2静脉滴注>1 h,联合卡铂AUC 2静脉滴注>30 min,第1、8、15天用药,21 d为1个周期,共6个周期。
本共识旨在为老年卵巢癌患者围手术期管理提供指导性意见,但并非为惟一的临床指南,不排除其他指南、共识、专家意见及建议的合理性。
利益冲突:所有作者均声明不存在利益冲突。
参考文献
(在框内滑动手指即可浏览)
[1] Sung H,Ferlay J,Siegel RL,et al.Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249.
[2] Duan Y,Han J,Yang L,et al. Real-world study of elderly patients with epithelial ovarian cancer:identifying and unmet needs[J]. Asian J Surg,2024,47(2):1306-1307.
[3] Mills S,Donnan P,Buchanan D,et al.Age and cancer type:associations with increased odds of receiving a late diagnosis in people with advanced cancer[J]. BMC Cancer,2023,23(1):1174.
[4] van Walree IC,van Soolingen NJ,Hamaker ME,et al. Treatment decision-making in elderly women with ovarian cancer:an age-based comparison[J]. Int J Gynecol Cancer,2019,29(1):158-165.
[5] Langstraat C,Aletti GD,Cliby WA. Morbidity,mortality and overall survival in elderly women undergoing primary surgical debulking for ovarian cancer:a delicate balance requiring individualization[J]. Gynecol Oncol,2011,123(2):187-191.
[6] Fourcadier E,Trétarre B,Gras-Aygon C,et al. Under-treatment of elderly patients with ovarian cancer:a population based study[J]. BMC Cancer,2015,15:937.
[7] Rausei S,Uccella S,D'Alessandro V,et al. Aggressive surgery for advanced ovarian cancer performed by a multidisciplinary team:A retrospective analysis on a large series of patients[J]. Surg Open Sci,2019,1(1):43-47.
[8] Mulligan K,Corry E,Donohoe F,et al.Multidisciplinary surgical approach to increase survival for advanced ovarian cancer in a tertiary gynaecological oncology centre[J]. Ann Surg Oncol,2024,31(1):460-472.
[9] Khassan T,Smitten E,Wood N,et al. MDT practice determines treatment pathway for patients with advanced ovarian cancer:A multi-centre observational study[J]. Eur J Surg Oncol,2023,49(8):1504-1510.
[10] Hernandez Torres C,Hsu T. Comprehensive geriatric assessment in the older adult with cancer:a review[J]. Eur Urol Focus,2017,3(4-5):330-339.
[11] Devoto L,Celentano V,Cohen R,et al. Colorectal cancer surgery in the very elderly patient:a systematic review of laparoscopic versus open colorectal resection[J]. Int J Colorectal Dis,2017,32(9):1237-1242.
[12] Fried LP,Tangen CM,Walston J,et al. Frailty in older adults:evidence for a phenotype[J]. J Gerontol A Biol Sci Med Sci,2001,56(3):M146-M156.
[13] Mitnitski AB,Mogilner AJ,Rockwood K. Accumulation of deficits as a proxy measure of aging[J]. Sci World J,2001,1:323-336.
[14] Ferrero A,Massobrio R,Villa M,et al. Development and clinical application of a tool to identify frailty in elderly patients with gynecological cancers[J]. Int J Gynecol Cancer,2023 Jul 24:ijgc-2023-004306.
[15] Zhang Q,Wang Z,Song M,et al. Effects of systemic inflammation and frailty on survival in elderly cancer patients:Results from the INSCOC study[J]. Front Immunol,2023,14:936904.
[16] Rodrigues MK,Marques A,Lobo DML,et al. Pre-frailty increases the risk of adverse events in older patients undergoing cardiovascular surgery[J]. Arq Bras Cardiol,2017,109(4):299-306.
[17] Montroni I,Ugolini G,Saur NM,et al. Predicting functional recovery and quality of life in older patients undergoing colorectal cancer surgery:real-world data from the international GOSAFE study[J]. J Clin Oncol,2023,41(34):5247-5262.
[18] Huisingh-Scheetz M,Walston J. How should older adults with cancer be evaluated for frailty? [J]. J Geriatr Oncol,2017,8(1):8-15.
[19] Liu C,Gao W,Meng W,et al. Can the Tilburg Frailty Indicator predict post-operative quality of recovery in patients with gynecologic cancer? A prospective cohort study[J]. Int J Gynecol Cancer,2023,33(5):761-769.
[20] Looijaard SMLM,Slee-Valentijn MS,Otten RHJ,et al. Physical and nutritional prehabilitation in older patients with colorectal carcinoma:a systematic review[J]. J Geriatr Phys Ther,2018,41(4):236-244.
[21] Stefani GP,Crestani MS,Scott LM,et al. Complementarity of nutritional assessment tools to predict prolonged hospital stay and readmission in older patients with solid tumors:A secondary analysis of a cohort study[J]. Nutrition,2023,113:112089.
[22] Zhang Q,Yu S,Li Q,et al. Preoperative nutritional status in elderly inpatients with gastrointestinal cancer and its linear association with frailty[J]. Nutr Cancer,2022,74(4):1376-1387.
[23] Zhang Y,Tan S,Wu G. ESPEN practical guideline:clinical nutrition in surgery[J]. Clin Nutr,2021,40(9):5071.
[24] Jin F,Chung F. Minimizing perioperative adverse events in the elderly[J]. Br J Anaesth,2001,87(4):608-624.
[25] Suidan RS,Ramirez PT,Sarasohn DM,et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian,fallopian tube,and peritoneal cancer[J]. Gynecol Oncol,2014,134(3):455-461.
[26] Kristensen SD,Knuuti J,Saraste A,et al. 2014 ESC/ESA Guidelines on non-cardiac surgery:cardiovascular assessment and management:The Joint Task Force on non-cardiac surgery:cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA)[J]. Eur Heart J,2014,35(35):2383-2431.
[27] Fleisher LA,Fleischmann KE,Auerbach AD,et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery:a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines[J]. J Am Coll Cardiol,2014,64(22):e77-e137.
[28] Kusne YN,Kosiorek HE,Buras MR,et al. Mortality and glycemic control among patients with diabetes mellitus and uterine or ovarian cancer[J]. Future Sci OA,2020,7(3):FSO670.
[29] Roth J,Sommerfeld O,Birkenfeld AL,et al. Blood sugar targets in surgical intensive care-management and special considerations in patients with diabetes[J]. Dtsch Arztebl Int,2021,118(38):629-636.
[30] Gröschel K,Harrer JU,Schminke U,et al. Ultrasound assessment of brain supplying arteries (transcranial)[J]. Ultraschall Med,2023,44(5):468-486.
[31] Mueller MM,Van Remoortel H,Meybohm P,et al.Patient blood management:recommendations from the 2018 Frankfurt Consensus Conference[J]. JAMA,2019,321(10):983-997.
[32] Peters J,Pendry K. Patient blood management:an update of current guidance in clinical practice[J]. Br J Hosp Med (Lond),2017,78(2):88-95.
[33] Key NS,Khorana AA,Kuderer NM,et al.Venous thromboembolism prophylaxis and treatment in patients with cancer:ASCO clinical practice guideline update[J]. J Clin Oncol,2020,38(5):496-520.
[34] Xu Y,Jia Y,Zhang Q,et al. Incidence and risk factors for postoperative venous thromboembolism in patients with ovarian cancer:Systematic review and meta-analysis[J]. Gynecol Oncol,2021,160(2):610-618.
[35] Liang S,Tang W,Ye S,et al. Incidence and risk factors of preoperative venous thromboembolism and pulmonary embolism in patients with ovarian cancer[J]. Thromb Res,2020,190:129-134.
[36] American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology.Prevention of venous thromboembolism in gynecologic surgery ACOG practice bulletin,number 232[J].Obstet Gynecol,2021,138(1):e1-e15.
[37] Guntupalli SR,Brennecke A,Behbakht K,et al. Safety and efficacy of Apixaban vs Enoxaparin for preventing postoperative venous thromboembolism in women undergoing surgery for gynecologic malignant neoplasm:a randomized clinical trial[J]. JAMA Netw Open,2020,3(6):e207410.
[38] Dranitsaris G,Shane LG,Woodruff S. Low-molecular-weight heparins for the prevention of recurrent venous thromboembolism in patients with cancer:A systematic literature review of efficacy and cost-effectiveness[J]. J Oncol Pharm Pract,2019,25(1):68-75.
[39] Shang MM,Yan R,Wang XL,et al. Comparison of 2013 and 2009 versions of Caprini risk assessment models for predicting VTE in Chinese cancer patients:a retrospective study[J]. J Thromb Thrombolysis,2020,50(2):446-451.
[40] Fotopoulou C,Planchamp F,Aytulu T,et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery[J]. Int J Gynecol Cancer,2021,31(9):1199-1206.
[41] Noh JJ,Lee YY. Where is ERAS in the management of advanced ovarian cancer?:between myths and truths[J]. J Gynecol Oncol,2022,33(5):e79.
[42] Sánchez-Iglesias JL,Gómez-Hidalgo NR,Pérez-Benavente A,et al. Importance of enhanced recovery after surgery (ERAS) protocol compliance for length of stay in ovarian cancer surgery[J]. Ann Surg Oncol,2021,28(13):8979-8986.
[43] Reuter S,Woelber L,Trepte CC,et al. The impact of Enhanced Recovery after Surgery (ERAS) pathways with regard to perioperative outcome in patients with ovarian cancer[J]. Arch Gynecol Obstet,2022,306(1):199-207.
[44] Martínez-Calle N,Hidalgo F,Alfonso A,et al. Implementation of a management protocol for massive bleeding reduces mortality in non-trauma patients:Results from a single centre audit[J]. Med Intensiva,2016,40(9):550-559.
[45] Moslemi-Kebria M,El-Nashar SA,Aletti GD,et al. Intraoperative hypothermia during cytoreductive surgery for ovarian cancer and perioperative morbidity[J]. Obstet Gynecol,2012,119(3):590-596.
[46] Kaufner L,Niggemann P,Baum T,et al. Impact of brief prewarming on anesthesia-related core-temperature drop,hemodynamics,microperfusion and postoperative ventilation in cytoreductive surgery of ovarian cancer:a randomized trial[J]. BMC Anesthesiol,2019,19(1):161.
[47] Feldheiser A,Conroy P,Bonomo T,et al. Development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgery[J]. J Int Med Res,2012,40(4):1227-1241.
[48] Sessler DI.Perioperative thermoregulation and heat balance[J]. Lancet,2016,387(10038):2655-2664.
[49] Anic K,Schmidt MW,Droste A,et al. Influence of anesthetic technique on survival after tumor debulking surgery of elderly patients with ovarian cancer:Results of a retrospective cohort study[J]. Oncol Lett,2022,24(4):361.
[50] Sessler DI.Perioperative thermoregulation and heat balance[J]. Lancet,2016,387(10038):2655-2664.
[51] Myles PS,Bellomo R,Corcoran T,et al. Restrictive versus liberal fluid therapy for major abdominal surgery[J]. N Engl J Med,2018,378(24):2263-2274.
[52] Yeniay H,Kuvaki B,Ozbilgin S,et al. Anesthesia management and outcomes of gynecologic oncology surgery[J]. Postgrad Med,2023,135(6):578-587.
[53] Onda T,Satoh T,Ogawa G,et al.Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian,tubal and peritoneal cancers in phase III randomised trial[J]. Eur J Cancer,2020,130:114-125.
[54] Simpson JC,Bao X,Agarwala A.Pain management in enhanced recovery after surgery (ERAS) protocols[J]. Clin Colon Rectal Surg,2019,32(2):121-128.
[55] Nelson G,Fotopoulou C,Taylor J,et al. Enhanced recovery after surgery (ERAS®) society guidelines for gynecologic oncology:Addressing implementation challenges - 2023 update[J]. Gynecol Oncol,2023 ,173:58-67.
[56] Dmytriiev D,Nazarchuk O,Melnychenko M,et al.Optimization of the target strategy of perioperative infusion therapy based on monitoring data of central hemodynamics in order to prevent complications[J]. Front Med (Lausanne),2022,9:935331.
[57] Zhang QF,Ding B, Chen MS,et al. Restrictive fluid therapy for high-complexity advanced ovarian cancer surgery:a single-center retrospective cohort study[J].Clin Exp Obstet Gynecol,2023,50(1):5.
[58] Muscaritoli M,Arends J,Aapro M. From guidelines to clinical practice:a roadmap for oncologists for nutrition therapy for cancer patients[J]. Ther Adv Med Oncol,2019,11:1758835919880084.
[59] Wischmeyer PE,Carli F,Evans DC,et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on nutrition screening and therapy within a surgical enhanced recovery pathway[J]. Anesth Analg,2018,126(6):1883-1895.
[60] Rinninella E,Fagotti A,Cintoni M,et al. Nutritional interventions to improve clinical outcomes in ovarian cancer:a systematic review of randomized controlled trials[J]. Nutrients,2019,11(6):1404.
[61] Lindemann K,Kleppe A,Eyjólfsdóttir B,et al.Prospective evaluation of an enhanced recovery after surgery (ERAS) pathway in a Norwegian cohort of patients with suspected or advanced ovarian cancer[J]. Int J Gynecol Cancer,2023,33(8):1279-1286.
[62] Tazreean R,Nelson G,Twomey R. Early mobilization in enhanced recovery after surgery pathways:current evidence and recent advancements[J]. J Comp Eff Res,2022,11(2):121-129.
[63] Rocher G,Gaillard T,Uzan C,et al. Does time-to-chemotherapy after primary complete macroscopic cytoreductive surgery influence prognosis for patients with epithelial ovarian cancer?A study of the FRANCOGYN Group[J]. J Clin Med,2021,10(5):1058.
[64] Hofstetter G,Concin N,Braicu I,et al. The time interval from surgery to start of chemotherapy significantly impacts prognosis in patients with advanced serous ovarian carcinoma - analysis of patient data in the prospective OVCAD study[J]. Gynecol Oncol,2013,131(1):15-20.
[65] Piedimonte S,Bernardini MQ,May T,et al. Treatment outcomes and predictive factors in patients ≥70 years old with advanced ovarian cancer[J]. J Surg Oncol,2022,125(4):736-746.
[66] NCCN. NCCN clinical practice guidelines in ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 2,2024[EB/OL].https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.














 苏公网安备32059002004080号
苏公网安备32059002004080号


