HR+/HER2+乳腺癌诊疗研究进展
DOI: 10.3969/j.issn.2095-1264.2022.06.01
文章编号: 2095-1264(2022)06-0685-05
作者简介:袁中玉,男,医学博士,教授,主任医师,研究方向:乳腺癌。
内分泌优先原则在靶向治疗时代仍然适用?
——SYSUCC-002研究解读
袁中玉
(中山大学肿瘤防治中心 内科,广东 广州,510060)
摘要: 激素受体(HR)阳性伴人表皮生长因子受体2(HER2)阳性乳腺癌(HR+/HER2+乳腺癌)是相对特殊的分子亚型,目前暂无HR+/HER2+晚期乳腺癌一线治疗方案选择的临床证据。为此,研究者开展了SYSUCC-002研究。SYSUCC-002研究是一项多中心、开放、随机、非劣效、Ⅲ期临床研究(NCT01950182),旨在比较内分泌治疗联合靶向治疗对比化疗联合靶向治疗两种治疗方式的有效性和安全性。该研究共入组392例HR+/HER2+晚期乳腺癌患者,按照1∶1随机分为内分泌治疗组(ET组,内分泌治疗+曲妥珠单抗)和化疗组(CT组,紫杉醇、卡培他滨或长春瑞滨+曲妥珠单抗)。研究的主要终点为无进展生存期(PFS),危险比(HR)的非劣效性上限为1.35。研究的主要终点结果显示,CT组和ET组患者的中位PFS分别为19.2个月和14.8个月,HR为0.88(95% CI: 0.71~1.09)(log-rank P=0.250)。研究的次要终点结果显示,两组患者OS无显著差异。进一步亚组分析显示,影响PFS的主要因素是无病间期(DFI),对于DFI≤24个月的患者采用CT方案PFS更优,而DFI>24个月的患者采用ET方案PFS更优(P=0.016)。对于HR+/HER2+晚期乳腺癌患者,曲妥珠单抗联合内分泌治疗的疗效不亚于曲妥珠单抗联合化疗,且毒性更低,有更好的耐受性。随着双靶时代的到来,内分泌优先原则这一治疗理念将继续指导临床医生作出决策。
关键词: 乳腺癌; 人表皮生长因子受体2阳性; 激素受体阳性; 一线治疗; 曲妥珠单抗联合内分泌治疗
中图分类号: R737.9 文献标识码: A
Is endocrine priority still relevant in the era of targeted therapy? ——SYSUCC-002 research interpretation
YUAN Zhongyu
(Medical Department, Sun Yat-sen University Cancer Center, Guangzhou, 510060, Guangdong, China)
Abstract: Hormone receptor (HR)-positive breast cancer with human epidermal growth factor receptor 2 (HER2)-positive breast cancer (HR+/HER2+ breast cancer) is a relatively specific molecular subtype. There is currently no clinical evidence for first-line treatment of HR+/HER2+ advanced breast cancer. For this purpose, the SYSUCC-002 study was conducted. SYSUCC002 is a multicenter, open, randomized, non-inferiority, phase Ⅲ clinical study (NCT01950182) designed to compare the efficacy and safety of endocrine therapy combined with targeted therapy versus chemotherapy combined with targeted therapy. 392 patients with HR+/HER2+ advanced breast cancer were randomized 1∶1 into endocrine therapy (ET) group [endocrine therapy + trastuzumab] and chemotherapy (CT) group (paclitaxel, capecitabine or vinrelbine + trastuzumab). The primary endpoint was progression-free survival (PFS) with a hazard ratio (HR) non-inferiority upper limit of 1.35. The primary endpoint results showed that the median PFS in the CT and ET groups were 19.2 months and 14.8 months, respectively, HR 0.88 (95% CI: 0.71~1.09) (log-rank P=0.250). Secondary endpoint results of the study showed no difference in OS between the two groups. Further subgroup analysis showed that the main factor affecting PFS was disease-free interval (DFI). For patients with DFI≤24 months, those in CT group had better PFS, whereas for patients with DFI>24 months, those in ET group had better PFS (P=0.016). Overall, for HR+/HER2+ advanced breast cancer patients, trastuzumab combined with endocrine therapy was no less effective than trastuzumab combined with chemotherapy, with lower toxicity and better tolerance. With the advent of the dual-target era, the treatment philosophy of endocrine-first principles will continue to guide clinicians' decision making.
Keywords:Breast cancer; Human epidermal growth factor receptor 2-positive; Hormone receptor-positive; First-line treatment; Trastuzumab combined with endocrine therapy
背景
近年来,乳腺癌的发病率已跃居恶性肿瘤首位,且呈逐年上升趋势,约30%的早期乳腺癌患者出现复发[1],约10%在初诊时即为晚期[2]。尽管晚期乳腺癌(advanced breast cancer, ABC)是不可治愈的,但合理的治疗可显著延长患者的生存时间,提高其生存质量。
HR+/HER2+乳腺癌指激素受体(hormone receptor, HR)和人表皮生长因子受体2(human epidermal growth factor receptor 2, HER2)均为阳性的乳腺癌,约占所有女性乳腺癌的10%[3-4],其中20%的ABC为HR+/HER2+亚型[5]。由于雌激素受体(estrogen receptor, ER)和HER2通路存在一定程度的交叉,不同HR状态/HER2+乳腺癌之间存在分子异质性,单纯阻断单一信号通路难以完全达到抑制肿瘤激活的作用[6]。针对ABC的治疗,目前至少有两点共识:①ABC属于全身性疾病,需采用综合治疗的策略和全程管理的治疗理念;②对于HR+/HER2+乳腺癌,内分泌、化疗和靶向治疗都是有效的治疗手段。对于HR+/HER2-、仅有骨及软组织转移或无症状内脏转移的ABC患者,应优先选择内分泌治疗(即内分泌优先的治疗原则),这是由于内分泌治疗毒性较低,患者维持有效时间较长,且先用内分泌治疗不损害患者总生存期(overall survival, OS)[7-9]。化疗主要用于对内分泌治疗不敏感或耐药、疾病进展迅速、有内脏转移且症状明显的ABC患者[10]。与单独化疗相比,化疗联合靶向治疗可显著延长ABC患者的OS[11-13],且使患者死亡风险下降25%[14]。此外,靶向治疗联合内分泌治疗也可显著提高患者疗效,延长无进展生存期(progression-free survival, PFS)[1, 15-16]。
目前临床实践中存在的问题是,对于HR+/HER2+ ABC患者,一线治疗是选择化疗联合靶向治疗,还是选择内分泌联合靶向治疗。有学者认为,HR和HER2共表达的患者对内分泌治疗耐药[2-3, 17-21],此类患者采用内分泌治疗受益较小[4],而化疗联合靶向治疗的疗效不受激素受体影响[5],因此首选化疗联合靶向治疗。另外,由于HR+乳腺癌对化疗耐药[7],化疗的有效率并不比内分泌治疗高[8],化疗联合赫赛汀治疗的有效率显著降低[9],而抗HER2治疗可延缓内分泌药物耐药性的产生[10],因此,这类患者首选内分泌治疗联合靶向治疗。
对于HR+/HER2+ ABC一线治疗方案究竟如何选择,目前暂无临床证据。为此,研究者开展了SYSUCC-002研究,直接比较内分泌治疗联合靶向治疗和化疗联合靶向治疗两种治疗方式的有效性和安全性。
1 方法
SYSUCC002研究[22]是一项在中国9家医院进行的开放、随机、多中心、非劣效Ⅲ期临床研究(NCT01950182),旨在确定与曲妥珠单抗联合化疗相比,曲妥珠单抗联合内分泌治疗是否同样有效且不良反应更少。研究对象为18岁及以上经病理证实的晚期乳腺癌女性患者,HR+和HER2+,ECOG评分0~1分,器官功能状态良好,预期存活时间≥12周,无病间期(disease-free interval, DFI)>12个月。将患者按照1∶1随机分为内分泌治疗组[ET组,采用雌激素受体调节剂(oestrogen-receptor modulator, ORM)或芳香化酶抑制剂(aromatase inhibitor, AI)+曲妥珠单抗治疗]和化疗组(CT组,采用紫杉醇、卡培他滨或长春瑞滨+曲妥珠单抗治疗)。主要研究终点为PFS,危险比(hazard ratio, HR)的非劣效性上限为1.35。
2 结果
2013年9月16日—2019年12月28日,共纳入392例患者,ET组和CT组各196例,两组ER阳性和孕激素受体(progesterone receptor, PR)阳性的患者均为157例(80.1%),两组既往接受过抗HER2治疗的患者分别为41例(20.9%)和48例(24.5%)。
研究的主要终点结果显示,CT组和ET组患者的中位PFS分别为19.2个月和14.8个月,两组的HR为0.88(95% CI: 0.71~1.09)(log-rank P=0.250)(图1)。研究的次要终点结果显示,两组OS的HR值为0.82(95% CI: 0.65~1.04)(P=0.090)。进一步亚组分析显示,影响PFS的主要因素是DFI,对于DFI≤24个月的患者采用CT方案PFS更优,而DFI>24个月的患者采用ET方案PFS更优(P=0.016)。
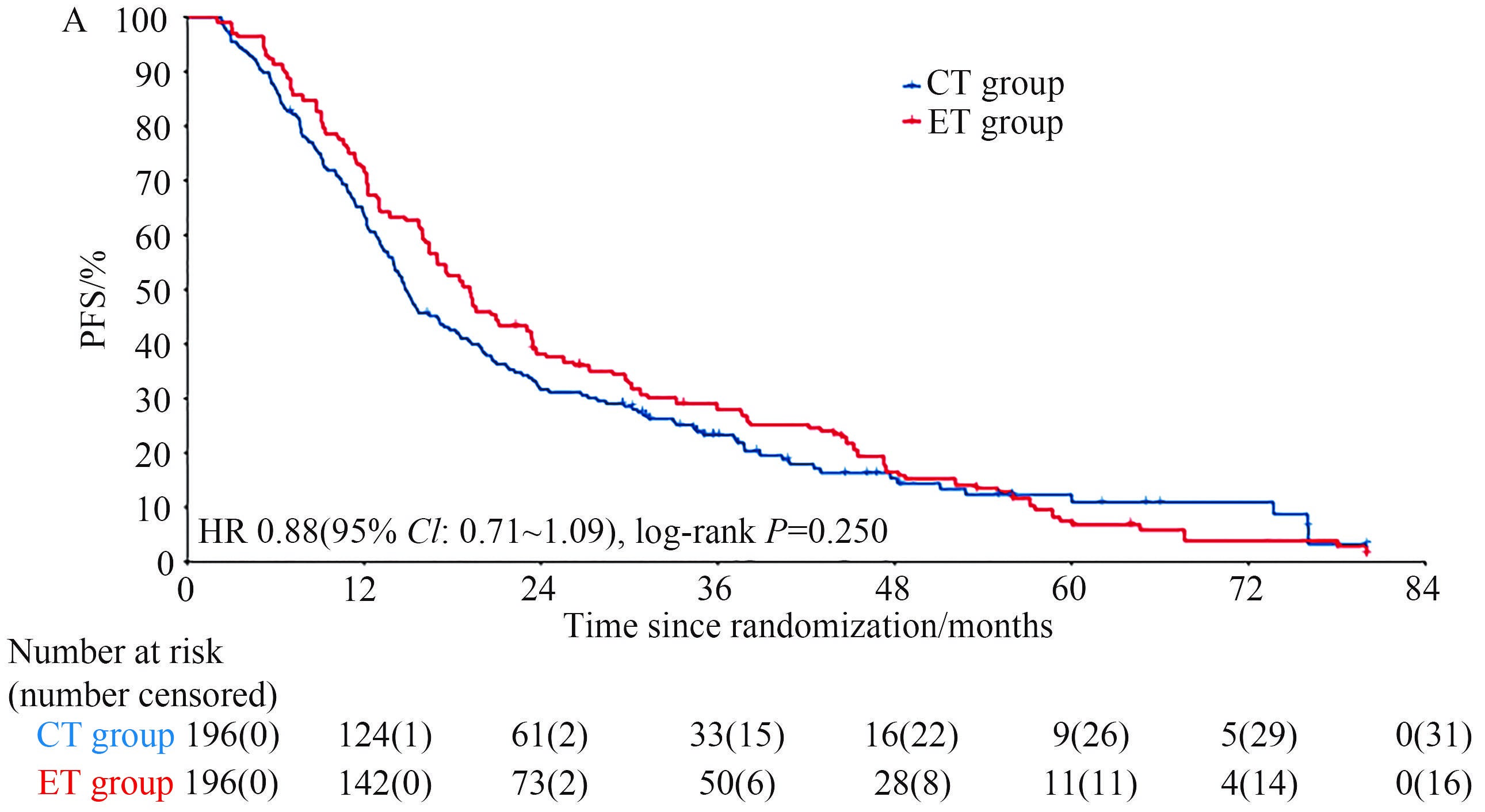
图1 CT组和ET组患者的中位PFS
Fig.1 Median PFS of patients in CT and ET groups
就毒副反应而言,CT组患者化疗相关白细胞减少(29.1%)、恶心(44.9%)、呕吐(19.4%)及腹泻(30.1%)的发生率更高,而ET组患者乏力(15.8%)、关节疼痛(16.8%)的发生率更高,但ET组3级及以上不良反应的发生率更低。
3 结论
对于HR+/HER2+ ABC患者,曲妥珠单抗联合内分泌治疗的疗效不亚于曲妥珠单抗联合化疗,且毒性更低,可为患者提供更方便的治疗,并具有更好的耐受性。
4 实践意义
SYSUCC002研究首次证实抗HER2治疗联合内分泌治疗可作为HR+/HER2+ ABC患者的一线治疗方案,这种去化疗模式与联合化疗疗效相似,且具有更高的安全性,尤其是3~4级治疗相关的血液学和胃肠道不良反应更少。值得注意的是,亚组分析显示,对于DFI≤24个月的患者使用CT方案PFS更优,而DFI>24个月的患者使用ET方案PFS更优。DFI≤24个月的患者接受抗HER2治疗联合内分泌治疗结局较差的原因可能是,患者在内分泌治疗开始后的24个月内出现内分泌耐药以及复发和/或转移,提示早期复发(DFI≤24个月)的患者可能不是抗HER2联合内分泌治疗的合适人群,这一治疗异质性结果还需要进一步研究证实。
对于SYSUCC002研究,有几点需要关注。首先,曲妥珠单抗和帕妥珠单抗双重阻断HER2治疗已被证实比抗HER2单药治疗能更进一步改善患者的生存结局,并被推荐为抗HER2治疗一线方案。然而,SYSUCC002研究是在2013年注册的,当时中国尚未批准帕妥珠单抗上市,因此未采用双靶治疗。参照CLEOPATRA研究[23]和PERTAIN研究[24]结果,SYSUCC002研究的结论在双靶治疗时代极有可能也是有效的,但还需要前瞻性临床试验来证实。随着新药研发的进展,必然会有许多高效的新药被纳入经典的传统治疗方案,指南推荐也会不断更新,但这些经典方案所呈现的治疗理念仍然具有重要的启示意义(方案会过时,但理念不会过时)。因此,内分泌优先原则这一治疗理念在双靶时代应该也同样适用。
其次,SYSUCC002研究方案的设计和批准早于细胞周期蛋白依赖性激酶(cyclin-dependent kinases, CDK)4/6抑制剂时代,因此ET组未使用CDK4/6抑制剂。CDK4/6抑制剂联合内分泌治疗在ER+乳腺癌中的治疗中发挥了重要作用。在多项Ⅰ/Ⅱ期临床试验中,CDK4/6抑制剂、内分泌治疗和抗HER2药物联合治疗显示出了很有前景的疗效[25-26]。在HR+/HER2+ ABC患者的一线治疗中,这种新型联合治疗方案是否优于化疗联合抗HER2治疗,还需要随机对照试验进一步研究。目前,已有多项抗HER2治疗联合内分泌治疗的研究在临床中积极开展[27-28]。
第三,虽然SYSUCC002研究中的化疗方案未统一,但曲妥珠单抗联合长春瑞滨或卡培他滨已被各国指南普遍接受为一种治疗选择,并取得了相似的疗效[29-32]。在不同化疗方案疗效相似的情况下,我们的研究重点是比较化疗与内分泌治疗的疗效。
随着以SYSUCC002为代表的中国HR+/HER2+乳腺癌临床试验数据的出炉,未来的治疗选择将会更加有据可依,从而使中国广大HR+/HER2+晚期乳腺癌患者从中获益。
[1] JEMAL A, SIEGEL R, WARD E, et al. Cancer statistics, 2007 [J]. CA Cancer J Clin, 2007, 57(1): 43-66. DOI: 10.3322/canjclin.57.1.43.
[2] DAWOOD S, BROGLIO K, GONZALEZ-ANGULO A M, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer [J]. J Clin Oncol, 2008, 26(30): 4891-4898. DOI: 10.1200/JCO. 2007.14.1168.
[3] SCHWARTZBERG L S, FRANCO S X, FLORANCE A, et al. Lapatinib plus letrozole as first-line therapy for HER-2+hormone receptor-positive metastatic breast cancer [J]. Oncologist, 2010, 15(2): 122-129. DOI: 10.1634/theoncologist.2009-0240.
[4] XUE C, WANG X, PENG R J, et al. Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China [J]. Cancer Sci, 2012, 103(9): 1679-1687. DOI: 10.1111/j. 1349-7006.2012.02339.x.
[5] DIXON A R, JACKSON L, CHAN S, et al. A randomised trial of second-line hormone vs single agent chemotherapy in tamoxifen resistant advanced breast cancer [J]. Br J Cancer, 1992, 66(2): 402-404. DOI: 10.1038/bjc.1992.277.
[6] CORTÉS J, SAURA C, BELLET M, et al. HER2 and hormone receptor-positive breast cancer—blocking the right target [J]. Nat Rev Clin Oncol, 2011, 8(5): 307-311. DOI: 10.1038/nrclinonc.2010.185.
[7] A randomized trial in postmenopausal patients with advanced breast cancer comparing endocrine and cytotoxic therapy given sequentially or in combination. The Australian and New Zealand Breast Cancer Trials Group, Clinical Oncological Society of Australia [J]. J Clin Oncol, 1986, 4(2): 186-193. DOI: 10. 1200/JCO.1986.4.2.186.
[8] WILCKEN N, HORNBUCKLE J, GHERSI D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer [J]. Cochrane Database Syst Rev, 2003, 2003(2): CD002747. DOI: 10.1002/14651858.CD002747.
[9] BESLIJA S, BONNETERRE J, BURSTEIN H J, et al. Third consensus on medical treatment of metastatic breast cancer [J]. Ann Oncol, 2009, 20(11): 1771-1785. DOI: 10.1093/annonc/mdp261.
[10] SEIDMAN A D, FORNIER M N, ESTEVA F J, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification [J]. J Clin Oncol, 2001, 19(10): 2587-2595. DOI: 10.1200/JCO.2001.19.10.2587.
[11] SLAMON D J, LEYLAND-JONES B, SHAK S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 [J]. N Engl J Med, 2001, 344(11): 783-792. DOI: 10.1056/NEJM2001031
53441101.
[12] MARTY M, COGNETTI F, MARANINCHI D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group [J]. J Clin Oncol, 2005, 23(19): 4265-4274. DOI: 10.1200/JCO. 2005.04.173.
[13] LIAO C, YIN F, HUANG P, et al. A meta-analysis of randomized controlled trials comparing chemotherapy plus trastuzumab with chemotherapy alone in HER-2-positive advanced breast cancer [J]. Breast J, 2011, 17(1): 109-111. DOI: 10. 1111/j.1524-4741.2010.01034.x.
[14] RIEMSMA R, FORBES C A, AMONKAR M M, et al. Systematic review of lapatinib in combination with letrozole compared with other first-line treatments for hormone receptor positive(HR+) and HER2+ advanced or metastatic breast cancer(MBC) [J]. Curr Med Res Opin, 2012, 28(8): 1263-1279. DOI: 10.1185/03007995.2012.707643.
[15] JOHNSTON S, PIPPEN J Jr, PIVOT X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer [J]. J Clin Oncol, 2009, 27(33): 5538-5546. DOI: 10.1200/JCO.2009.23.3734.
[16] KAUFMAN B, MACKEY J R, CLEMENS M R, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study [J]. J Clin Oncol, 2009, 27(33): 5529-5537. DOI: 10.1200/JCO.2008.20.6847.
[17] GENNARI A, CONTE P, ROSSO R, et al. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies [J]. Cancer, 2005, 104(8): 1742-1750. DOI: 10.1002/cncr.21359.
[18] ANDRE F, SLIMANE K, BACHELOT T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period [J]. J Clin Oncol, 2004, 22(16): 3302-3308. DOI: 10.1200/JCO.2004.08.095.
[19] SHIGEMATSU H, KAWAGUCHI H, NAKAMURA Y, et al. Significant survival improvement of patients with recurrent breast cancer in the periods 2001-2008 vs. 1992-2000 [J]. BMC Cancer, 2011, 11: 118. DOI: 10.1186/1471-2407-11-118.
[20] GIORDANO S H, BUZDAR A U, SMITH T L, et al. Is breast cancer survival improving? [J]. Cancer, 2004, 100(1): 44-52. DOI: 10.1002/cncr.11859.
[21] TEVAARWERK A J, GRAY R J, SCHNEIDER B P, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years [J]. Cancer, 2013, 119(6): 1140-1148. DOI: 10.1002/cncr.27819.
[22] HUA X, BI X W, ZHAO J L, et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002) [J]. Clin Cancer Res, 2022, 28(4): 637-645. DOI: 10.1158/1078-0432.CCR-21-3435.
[23] BASELGA J, CORTÉS J, KIM S B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer [J]. N Engl J Med, 2012, 366(2): 109-119. DOI: 10.1056/NEJMoa1113216.
[24] RIMAWI M, FERRERO J M, DE LA HABA-RODRIGUEZ J, et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial [J]. J Clin Oncol, 2018, 36(28): 2826-2835. DOI: 10.1200/JCO.2017.76.7863.
[25] MARCOM P K, ISAACS C, HARRIS L, et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers [J]. Breast Cancer Res Treat, 2007, 102(1): 43-49. DOI: 10.1007/s10549-006-9307-8.
[26] THANOPOULOU E, KHADER L, CAIRA M, et al. Therapeutic strategies for the management of hormone receptor-positive, human epidermal growth factor receptor 2-positive (HR+/HER2+) breast cancer: a review of the current literature [J]. Cancers, 2020, 12(11): 3317. DOI: 10.3390/cancers12113317.
[27] OUYANG Q C, XIONG H, YAN M, et al. Abstract P2-13-32: Pyrotinib in combination with letrozole for estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: A multicenter, single-arm, phase II trial [J]. Cancer Research, 2022, 82(4_Supplement): P2-13-32-P2-13-32.
[28] WANG Y, ZHAO J, YUAN Z, et al. Abstract P2-13-35: Pyrotinib combined with fulvestrant in women with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer: A single-arm phase II clinical trial [J]. Cancer Research, 2022, 82(4_Supplement): P2-13-35-P2-13-35.
[29] PRITCHARD K I, SOUSA B. ABC 1 (1st International consensus guidelines for advanced breast cancer): a positive step [J]. Breast, 2012, 21(3): 225-226. DOI: 10.1016/j.breast.2012. 05.010.
[30] CARDOSO F, HARBECK N, FALLOWFIELD L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [J]. Ann Oncol, 2012, 23(Suppl 7): vii11-vii19. DOI: 10.1093/annonc/mds232.
[31] ANDERSSON M, LIDBRINK E, BJERRE K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study [J]. J Clin Oncol, 2011, 29(3): 264-271. DOI: 10.1200/JCO.2010.30.8213.
[32] PIVOT X, MANIKHAS A, ŻURAWSKI B, et al. CEREBEL (EGF111438): A phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer [J]. J Clin Oncol, 2015, 33(14): 1564-1573.
本文引用格式:袁中玉. 内分泌优先原则在靶向治疗时代仍然适用?[J]. 肿瘤药学, 2022, 12(6): 685-689. DOI: 10.3969/j.issn.2095-1264.2022.06.01.
Cite this article as: YUAN Zhongyu. Is endocrine priority still relevant in the era of targeted therapy?——SYSUCC-002 research interpretation[J]. Anti-tumor Pharmacy, 2022, 12(6): 685-689. DOI: 10.3969/j.issn.2095-1264.2022.06.01.











 苏公网安备32059002004080号
苏公网安备32059002004080号