Claudin18作为细胞间紧密连接的重要结构蛋白,其亚型Claudin18.2(CLDN18.2)是一种CD20样分化的蛋白,尽管在正常组织中表达高度受限,在多种原发性恶性肿瘤发生发展过程中往往出现异常高表达,尤其好发于消化系统恶性肿瘤,包括胃癌、胰腺癌、食管癌等。近年来,多项研究的成功与探索使其成为继HER2之后,胃癌治疗领域的一颗璀璨的“明星靶点”。
在今年ASCO年会上,一众Claudin18.2治疗相关研究结果揭晓,涵盖胃癌、胰腺癌、胆道癌等实体肿瘤,采用单克隆抗体、双特异性抗体、抗体偶联药物(ADC)、嵌合抗原受体T细胞免疫疗法(CAR-T)等新型疗法,引领精准治疗新风向。这些新方法可能潜在地将CLDN18.2靶向治疗的益处扩展到更广泛的肿瘤类型以及表达较低水平CLDN18.2的肿瘤。
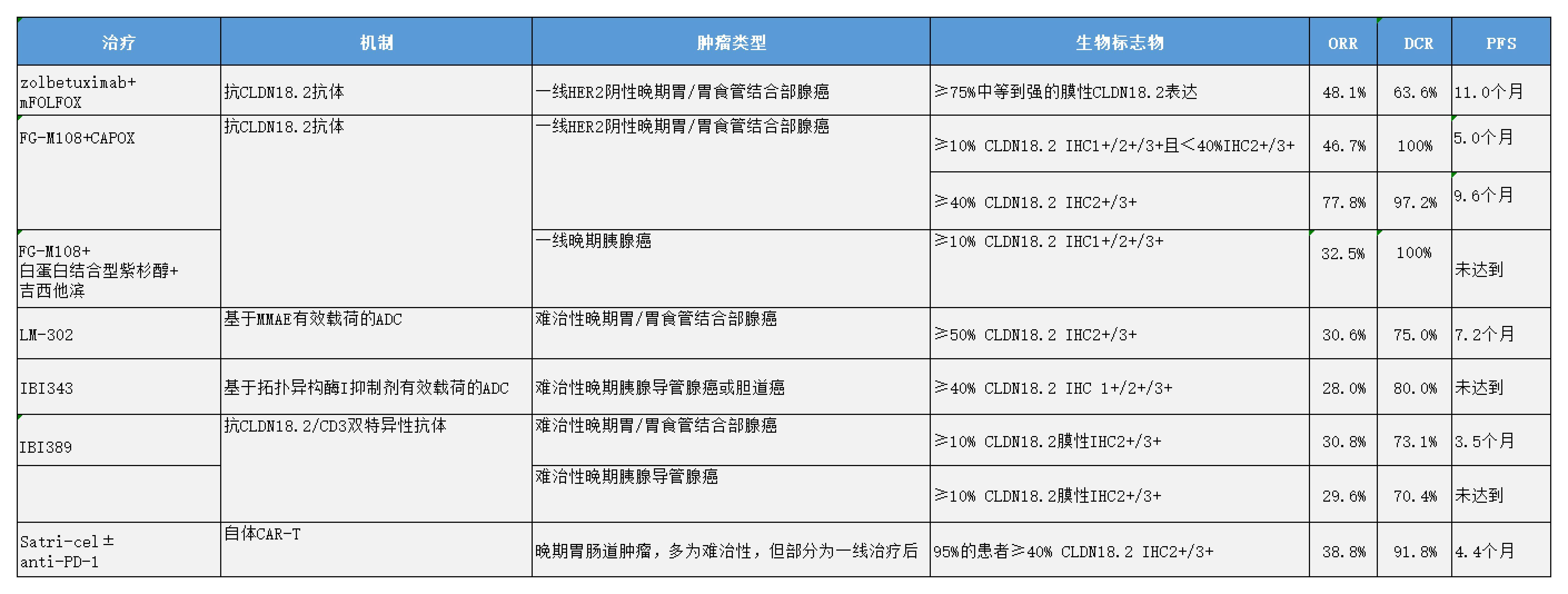
Zolbetuximab用于CLDN18.2阳性胃食管腺癌
Zolbetuximab
Zolbetuximab是一种CLDN18.2单克隆抗体,Ⅲ期SPOTLIGHT和GLOW研究证明了特异性针对CLDN18.2的抗体Zolbetuximab联合化疗用于晚期胃和胃食管结合部(GEJ)腺癌一线治疗的疗效。两项研究的入组人群肿瘤细胞中≥75%显示出中等到强的膜性CLDN18.2表达[1, 2]。2024 ASCO年会更新了SPOTLIGHT研究的最终数据[3],Zolbetuximab联合mFOLFOX对比安慰剂联合mFOLFOX,仍然显著提高了无进展生存(PFS)和总生存(OS),中位PFS分别为11.0个月 vs 8.9个月(HR 0.73,95% CI 0.59~0.91,P= 0.0024),中位OS分别为18.2个月vs 15.6个月(HR 0.78,95% CI 0.64~0.95,P= 0.0075)。客观缓解率(ORR)在两组中相似(48.1% vs 47.5%)。基于SPOTLIGHT和GLOW,Zolbetuximab已于2024年3月在日本获批晚期胃癌一线治疗适应症,用于治疗CLDN18.2阳性、不可切除、晚期或复发性胃癌患者,这也是目前全球首个被批准的CLDN18.2靶向疗法。
FG-M108用于CLDN18.2阳性胃食管癌和胰腺癌
FG-M108
另一CLDN18.2单克隆抗体——FG-M108的设计旨在增加抗体依赖性细胞毒性(ADCC),其通过增强型的ADCC效应充分调动患者集体的免疫细胞来杀伤肿瘤细胞。在一项Ⅰ/Ⅱa期研究中[4],未经治疗的晚期胃或GEJ腺癌患者接受了FG-M108联合CAPOX治疗。在36例入组患者中,≥40%肿瘤细胞CLDN18.2免疫组化(IHC)2+/3+。研究结果显示,该方案的ORR为77.8%,疾病控制率(DCR)为97.2%,而在15例IHC 1+/2+/3+≥10%且IHC 2+/3+<40%的CLDN18.2表达患者中,ORR为46.7%,DCR为100%。在40例未经治疗的晚期胰腺癌患者中,FG-M108联合吉西他滨和白蛋白结合型紫杉醇治疗,ORR为32.5%,DCR为100%[5]。研究者表示,该联合用药方案的耐受性良好,目前该研究正在进行中。
CLDN18.2阳性晚期实体瘤的新型治疗策略
除CLDN18.2单克隆抗体外,还包括抗体-药物偶联物(ADCs)、双特异性抗体和嵌合抗原受体T细胞(CAR-T)治疗等新型治疗策略。ADC药物兼具化疗与靶向治疗优势,较化疗具有肿瘤特异性,且较靶向药物具备更强的细胞毒性,具有良好的治疗潜力。
LM-302
LM-302是一种靶向CLDN18.2的ADC药物,由人类化抗CLDN18.2抗体与毒素载荷甲基澳瑞他汀E(MMAE)偶联而成。本次ASCO年会中披露了其在胃/GEJ癌和胆道癌中的研究数据。在一项Ⅰ/Ⅱ期研究中[6],LM-302在36例难治性晚期胃或GEJ癌患者中(≥50% CLDN18.2 IHC 2+/3+),观察到11例部分缓解(PR)和16例疾病稳定(SD);ORR为30.6%,DCR为75.0%。中位PFS为7.2个月,中位OS未达到,6个月的OS率为95.0%。在胆道癌(BTC)领域,一项多中心、两阶段、随机 Ⅰb/Ⅱ 期临床试验,在剂量递增阶段,招募了6例患者,分别接受 LM302(1.6mg/kg 或 1.8mg/kg Q2W)和卡度尼利单抗(6mg/kg Q2W)。6 例可评估疗效的患者中,3 例(CLDN18.2 表达率分别为 30%、40%、80%)获得 PR,Ⅰb 期 BOR 为 50%。LM-302 的 RP2D 为 1.8mg/kg[7]。
IBI343
另一种ADC药物——IBI343,由一个单克隆CLDN18.2抗体与拓扑异构酶I抑制剂偶联,其与表达CLDN18.2的肿瘤细胞结合后,可发生CLDN18.2依赖性ADC内化,并释放毒素药物引起DNA损伤,导致肿瘤细胞凋亡。在一项Ⅰ期试验中[8],IBI343在25例难治性晚期胰腺或胆道癌患者中(≥40% CLDN18.2 IHC 1+/ 2+/3+),ORR和DCR分别为28.0%和80.0%。
IBI389
IBI389是一款抗CLDN18.2的T细胞衔接双特异性抗体,通过连接T细胞受体复合体中的CD3和肿瘤细胞表面的CLDN18.2抗原,诱导免疫突触形成,刺激T细胞活化,促进细胞溶解蛋白的产生、炎性细胞因子的释放和T细胞进一步增殖,从而达到持续杀伤肿瘤细胞控制肿瘤生长的目的。一项Ⅰ期研究正在探索IBI389在难治性晚期实体瘤中的应用[9, 10]。在26例晚期胃或GEJ腺癌患者中(≥10% CLDN18.2 IHC 2+/3+),ORR为30.8%,DCR为73.1%,中位PFS为3.5个月[9]。在27例晚期胰腺导管腺癌患者中(≥10% CLDN18.2 IHC 2+/3+),ORR为29.6%,DCR为70.4%[10]。在120例入组患者中,有1例因消化道出血后的脑血管意外而死亡[9]。尽管60%的患者出现了细胞因子释放综合征(CRS),但多数为1~2级[9]。
satricabtagene autoleucel
CAR-T治疗为靶向CLDN18.2提供了另一种创新方法。Ⅰ期CT041-CG4006研究评估了satricabtagene autoleucel(satri-cel)——一种针对CLDN18.2的自体CAR-T产品。在98例消化道肿瘤患者中,75%为胃或GEJ癌[11, 12]。剂量扩展阶段包括61例接受satri-cel单药治疗的难治性消化道肿瘤患者,15例接受satri-cel联合PD-1单抗治疗的患者,5例患者在一线治疗进展后接受satri-cel治疗,以及2例既往接受过抗CLDN18.2抗体治疗的患者。CRS率为97%(均为1~2级),胃黏膜损伤率为8.2%。未观察到免疫效应细胞相关神经毒性综合征或治疗相关死亡。总体ORR为38.8%,DCR为91.8%,胃或GEJ癌的ORR为54.9%,胰腺癌为20%,胆道癌为50%,既往接受过抗CLDN18.2抗体治疗患者的ORR为50%。然而,中位PFS仅为4.4个月,中位OS为8.8个月,CAR-T细胞的中位持续时间仅为28天。
表1 2024ASCO年会中探索CLDN18.2靶向治疗临床试验总结
小结
以上研究表明,CLDN18.2不仅在胃和GEJ腺癌中颇具前景,其在其他晚期实体瘤中也是一个潜在靶点。新型CLDN18.2靶向治疗可能在CLDN18.2表达较低或对抗CLDN18.2抗体有抗性的肿瘤中具有疗效。需要进一步研究以确定CLDN18.2表达的适当阈值,联合疗法的潜在协同作用,以及CLDN18.2靶向治疗的最佳顺序[13]。
[1]Shitara K, Lordick F, Bang Y-J, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655–68. https://doi.org/10.1016/S0140-6736(23)00620-7.
[2]Shah MA, Shitara K, Ajani JA, Bang Y-J, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29:2133–41. https://doi.org/10.1038/s41591-023-02465-7.
[3]Shitara K, Van Cutsem E, Lordick F, Enzinger PC, Ilson DH, Shah MA et al. Final overall survival results from phase 3 SPOTLIGHT study evaluating zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) with claudin 18 isoform 2 (CLDN18.2)+, HER2–, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma. Poster presentation at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[4]Liu F, Gong J, Jin Z, Zhang M, Zhang S, Zhang Y et al. Safety and efficacy results from the phase I/IIa study of FG-M108 plus CAPOX as first-line (1L) treatment for patients with CLDN18.2+/HER2- locally advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Poster presentation at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[5]Jin Z, Zhang Y, Liu F, Zhang S, Gong J, Zhang M et al. FG-M108 plus nab-paclitaxel and gemcitabine (AG) as first-line (1L) treatment for patients with Claudin-18.2 (CLDN18.2) positive locally advanced unresectable or metastatic pancreatic cancer (PC): Preliminary results from the phase 1b study. Poster presentation at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[6]Bai C, Xue J, Zheng Y, Sun M, Ying J, Zhou F et al. A phase 1/2 study of LM-302, an anti-claudin 18.2 (CLDN18.2) antibody-drug conjugate in patients with advanced gastric/gastroesophageal junction cancer. Poster presentation at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[7]Jia Fan ,et al.Two stage, multi-center trial of cadonilimab and LM-302 for patients with CLDN18.2+ biliary tract cancer (BTC) that failed chemotherapy and PD-(L)1 antibody (ZSAB-Calm).2024ASCO Abstract #e16152.
[8]Yu X, Zhang J, Tazbirkova A, Yang J, Yue J, Sun Y et al. Safety and efficacy of IBI343 (anti-claudin18.2 antibody-drug conjugate) in patients with advanced pancreatic ductal adenocarcinoma or biliary tract cancer: Preliminary results from a phase 1 study. Poster presentation at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[9]Zheng L, Ruihong D, Jieer Y, Xu Q, Guo Z, Hu C et al. Safety and preliminary efficacy results of IBI389, an anti-CLDN18.2/CD3 bispecific antibody, in patients with solid tumors and gastric or gastro-esophageal tumors: A phase 1 dose escalation and expansion study. Oral session presented at 2024 ASCO Annual Meeting;2024 May 31 to June 4; Chicago, Illinois.
[10]Hao J, Zheng L, Ruihong D, Jieer Y, Xu Q, Wang L-W et al. Safety and efficacy of IBI389, an anti-CLDN18.2/CD3 bispecific antibody, in patients with advanced pancreatic ductal adenocarcinoma: Preliminary results from a phase 1 study. JCO. 2024;42:4011. Oral session presented at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[11]Qi C, Liu C, Gong J, Li J, Liu D, Wang X et al. Claudin18.2-targeted chimeric antigen receptor T cell-therapy for patients with gastrointestinal cancers: Final results of CT041-CG4006 phase 1 trial. JCO. 2024;42:2501. Oral session presented at 2024 ASCO Annual Meeting; 2024 May 31 to June 4; Chicago, Illinois.
[12]Qi C, Liu C, Gong J, Liu D, Wang X, Zhang P, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial final results. Nat Med. 2024. https://doi.org/10.1038/s41591-024-03037-z.
[13]Zhou KI, Strickler JH, Chen H. Targeting Claudin-18.2 for cancer therapy: updates from 2024 ASCO annual meeting. J Hematol Oncol. 2024;17(1):73. Published 2024 Aug 26. doi:10.1186/s13045-024-01595-w.
排版编辑:肿瘤资讯-AS











 苏公网安备32059002004080号
苏公网安备32059002004080号


