在刚刚落幕的2024年美国临床肿瘤学会(ASCO)年会上,多项国产抗肿瘤创新药研究成功跻身“重磅研究摘要”(Late-Breaking Abstract, LBA)之列,彰显了重要的临床价值和开创性意义,其中就包括KRAS G12C抑制剂氟泽雷塞(Fulzerasib)联合西妥昔单抗一线治疗KRAS G12C突变晚期非小细胞肺癌(NSCLC)的KROCUS研究(摘要号LBA8511),这也是KRAS G12C抑制剂联合EGFR抑制剂用于一线NSCLC治疗的临床数据“全球首发”[1]。
KROCUS是一项由欧洲研究者开展、面向欧洲患者的多中心“出海”研究,不仅证实了氟泽雷塞联合西妥昔单抗方案一线使用的良好疗效和安全性,也对KRAS G12C抑制剂一线联合方案的未来临床探索提供了更多可能。值此机会,本次邀请到广东省人民医院吴一龙教授、领衔开展KROCUS研究的西班牙专家Rafael Rosell教授、在ASCO年会现场口头报告研究成果的意大利专家Vanesa Gregorc教授共同探讨研究成果及其意义。
KROCUS研究“强效缩瘤”,满足KRAS G12C突变晚期NSCLC患者精准治疗需求
KRAS突变是晚期NSCLC中常见的癌基因突变之一,而KRAS G12C突变在众多KRAS突变类型中占比最高,在欧美国家非鳞NSCLC患者中整体检出率可达10%-13%。吴一龙教授介绍道,近年来的大样本测序研究数据显示,我国NSCLC患者携带KRAS G12C突变的比例约为2%-3%[2]。
近年来,NSCLC靶向治疗快速发展,KRAS G12C突变晚期NSCLC患者的精准治疗需求也亟待满足。Vanesa Gregorc教授指出,既往KRAS突变被视为“不可成药”靶点,KRAS G12C突变患者只能被迫接受化疗、免疫治疗等非靶向疗法。目前,KRAS G12C抑制剂在晚期NSCLC二线治疗中已经取得显著进展并有相应药物在国外获批,但KRAS G12C抑制剂一线联合治疗方案仍在探索中。KROCUS研究正致力于解决这一问题。
既往临床前研究显示,由EGFR等受体酪氨酸激酶(RTKs)介导的RAS通路反馈性激活是KRAS抑制剂耐药和疗效有限的原因之一;而KRAS抑制剂还可能通过下调MIG6水平导致EGFR表达上调,进一步促进EGFR介导的耐药[4-6]。因此KROCUS研究在设计思路上,采用了同时抑制EGFR和KRAS G12C的联合用药策略,以期达到更好的治疗疗效并延缓治疗耐药的发生。
截至2024年4月,KROCUS研究共入组40例KRAS G12C突变晚期一线NSCLC患者,在本次ASCO年会上公布的疗效分析数据显示:33例接受过至少一次治疗后肿瘤评估的患者中,氟泽雷塞联合西妥昔单抗治疗的客观缓解率(ORR)高达81.8%,含1例完全缓解(CR)和2例靶病灶缩小100%的部分缓解(PR),疾病控制率(DCR)高达100%,多数患者的肿瘤缩小幅度超过50%,且88%患者仍在继续接受治疗,中位缓解持续时间(mDoR)尚未达到。
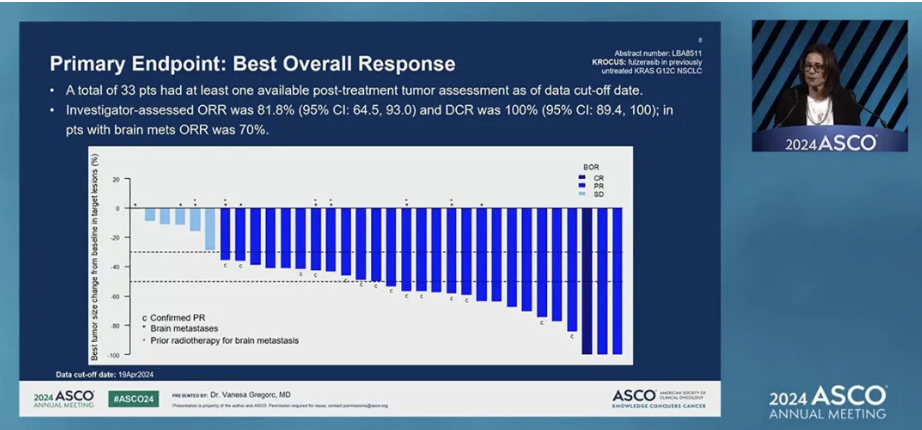 图1.KROCUS研究最佳治疗应答评估结果
图1.KROCUS研究最佳治疗应答评估结果
领衔开展KROCUS研究的Rafael Rosell教授和Vanesa Gregorc教授分别介绍了临床试验中令人印象深刻的两例患者案例,首例患者基线时已有脑转移,但在接受氟泽雷塞联合西妥昔单抗方案治疗后,脑转移灶缩小,肿瘤评估达到客观缓解、无需再行放疗,体现了氟泽雷塞良好的入脑能力和颅内疗效;另一例患者则在确诊时已有脑、骨、软组织等全身多处转移,且转移灶导致了严重并发症,患者在入组研究接受联合治疗后实现了快速且深度的疾病缓解,反映了联合治疗的出色疗效。
出色安全性为联合治疗“上分”,或在未来实现“去化疗”目标
KROCUS研究在安全性方面同样表现出色,Rafael Rosell教授介绍,氟泽雷塞联合西妥昔单抗方案治疗期间,患者未报告≥4级的治疗相关不良事件(TRAEs),联合治疗的安全性优势对比KRAS G12C突变患者既往常用的化疗更为明显,有望在未来实现“去化疗”式治疗,也让研究者们更有信心将氟泽雷塞联合西妥昔单抗方案推进至临床III期研究进一步评估。
吴一龙教授则提及了今年在《新英格兰医学杂志》发表的FLAURA2研究评论文章[3],指出从既往治疗我国EGFR突变NSCLC患者的经验来看,我国患者普遍不倾向于接受化疗。Vanesa Gregorc教授也表示:“KRAS G12C突变患者既往接受一线化疗是不得已之举,临床医生和患者都更倾向于使用疗效更好、副作用更小的靶向药物替代化疗,KROCUS研究在KRAS G12C突变晚期NSCLC一线治疗中的探索具有“先驱性”,氟泽雷塞联合西妥昔单抗有望实现一线治疗的目标”。
Vanesa Gregorc教授还专门指出,既往已经报道的KRAS抑制剂治疗常见的肝酶升高、腹泻等不良反应在KROCUS研究中均非常少见,患者报告发生率最高的治疗相关不良反应(TRAE)是皮疹(55.6%,但仅有1例3级),这对氟泽雷塞与西妥昔单抗联合治疗的应用非常有利。
 图2.KROCUS研究安全性数据总览
图2.KROCUS研究安全性数据总览
单药治疗与联合方案齐头并进,氟泽雷塞即将惠及中国患者
除了KROCUS联合研究,氟泽雷塞单药治疗KRAS G12C突变晚期NSCLC也有良好表现。
在2023年欧洲肿瘤内科学会亚洲大会(ESMO Asia)上,吴一龙教授团队的林嘉欣教授口头汇报了氟泽雷塞针对我国NSCLC患者的一项临床II期注册研究。初步研究结果显示,氟泽雷塞治疗KRAS G12C突变晚期NSCLC的确认ORR(cORR)为46.6%,DCR为90.5%,中位无进展生存期(mPFS)为8.3个月。
吴一龙教授表示,与此前获美国FDA批准的两款KRAS G12C抑制剂(Sotorasib, Adagrasib)相比,氟泽雷塞在临床II期研究中的ORR、mPFS两项关键疗效指标均更有优势,且治疗安全性同样良好。基于该研究结果,氟泽雷塞已获得国内治疗KRAS G12C突变晚期NSCLC的突破性疗法认定,并获国家药品监督管理局(NMPA)药品审评中心(CDE)受理,并纳入优先审评,期待其能够尽快获批惠及患者。
此外,氟泽雷塞还有与其他各类治疗药物联合使用、为患者带来更多获益的广泛可能性,如吴一龙教授和Vanesa Gregorc教授都认为,针对同时存在KRAS G12C突变和PD-L1高表达的患者,或可探索氟泽雷塞与PD-L1抑制剂的联合治疗,届时有望对药物排兵布阵进行优化、重新定位免疫治疗用法,而氟泽雷塞良好的疗效和安全性是实现上述目标的必要前提。
Professor of Oncology, PhD supervisor
Winner of Outstanding Science Achievement from IASLC
Vice President of Chinese Medical Doctors Association (CMDA)
President of Guangdong Medical Doctors Association (GDMDA)
Chief expert of Guangdong Provincial People's Hospital (GDPH)
Honorary Director, Guangdong Province Lung Cancer Research Institute (GLCI)
Chair of China Thoracic Oncology Group (CTONG)
Highly cited scientists in clinical medicine from 2018 to 2023
Chairman of Lung Oncology Society, Guangdong Medical Association
Past President Chinese Society of Clinical Oncology (CSCO), Chairman of the steering committee
Since 2021 to present
Affiliation: Istituto di Candiolo - IRCCS Fondazione del Piemonte per l'Oncologia (IT)
2021-to present Deputy Scientific Director
2021-to presentDirector of Medical Area
2021- to presentDirector of Medical Oncology Department
Since 2003 to 2021
Affiliation: IRCCS Ospedale San Raffaele, Milano (IT)
2021-11/2021Coordinator of Cancer Center Medical Area
2020-11/2021Director of the Strategic Diagnostic-Therapeutic Oncological Innovation Program
2015-2021Director of Thoracic Oncology, Melanoma and Head and Neck Unit at Medical Oncology Department
2003-2015Staff Physician of Thoracic Oncology, Melanoma and Head and Neck Unit at Medical Oncology Department
Catalan Institute of Oncology (ICO) – Badalona, Barcelona, Spain
Chief Medical Officer
Dr Rosell Oncology Institute, Quiron Dexeus Univ Hospital – Barcelona, Spain
Founder, Chairman & Chief Scientific Officer
Pangaea Biotech SL – Barcelona, Spain
Founder & President
Molecular Oncology Research Foundation (MORe Foundation) – Barcelona, Spain
Founder, Director of International Relations & Projects
Spanish Lung Cancer Group – Barcelona, Spain
Associate Professor/Lecturer, Medical Oncology
Autonomous University of Barcelona – Barcelona, Spain
Member:
International Association for the Study of Lung Cancer (IASLC) Chair, 2005 World Conference on Lung Cancer
European Society for Medical Oncology (ESMO) Member, Scientific Program Sub-Committee (NSCLC, metastatic)for ESMO Annual Congress
European Organization for Research and Treatment of Cancer (EORTC) Member of the EORTC Protocol Review Committee
European Thoracic Oncology Platform (ETOP) Foundation Council and Steering Committee Member
American Society of Clinical Oncology (ASCO) Member of Scientific Program Committee 2002-2004
Spanish Lung Cancer Group, Former President (1991-2014)
International Lung Adenocarcinoma Consensus Classification Panel (ILACCP)
Catalan Society of Oncology, Former President
Spanish Society of Medical Oncology Spanish Society of Cancer Research (ASEICA), Former President
American Association for Cancer Research
Honorary Member of Argentina Association of Clinical Oncology (AAOC)
Italian Association of Thoracic Oncology (AIOT)
Bonnie J Addario Lung Cancer Foundation, member Medical Advisory Board
International Association for the Study of Lung Cancer (IASLC)
European Cancer Concord (ECC) Founding member
[1]Gregorc V, et al. KROCUS: A phase II study investigating the efficacy and safety of fulzerasib (GFH925) in combination with cetuximab in patients with previously untreated advanced KRAS G12C mutated NSCLC[J]. Journal of Clinical Oncology, 2024, 42(suppl_17) : LBA8511.
[2]Lim T K H, Skoulidis F, Kerr K M, et al. KRAS G12C in advanced NSCLC: prevalence, co-mutations, and testing[J]. Lung Cancer, 2023, 184: 107293.
[3]Zhou Q, Meng X, Sun L, et al. LBA12 Efficacy and safety of IBI351 (GFH925), a selective KRASG12C inhibitor, monotherapy in patients (pts) with advanced non-small cell lung cancer (NSCLC): Initial results from a registrational phase II study[J]. Annals of Oncology, 2023, 34(Supplement 4): S1662.
[4]Ryan M B, Fece de la Cruz F, Phat S, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRASG12C inhibition[J]. Clinical Cancer Research, 2020, 26(7): 1633-1643.
[5]Amodio V, Yaeger R, Arcella P, et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer[J]. Cancer Discovery, 2020, 10(8): 1129-1139.
[6]Chen N, Tyler L C, Le A T, et al. MIG6 mediates adaptive and acquired resistance to ALK/ROS1 fusion kinase inhibition through EGFR bypass signaling[J]. Molecular Cancer Therapeutics, 2024, 23(1): 92-105.
排版编辑:肿瘤资讯-Rex











 苏公网安备32059002004080号
苏公网安备32059002004080号


