参考资料
[1]Wang Y, Yang X, Li NJ, Xue JX. Leptomeningeal metastases in non-small cell lung cancer: Diagnosis and treatment. Lung Cancer. 2022;174:1-13. doi:10.1016/j.lungcan.2022.09.013
[2]陆志琴,蔡婧,曾治民,刘安文.敏感驱动基因阳性非小细胞肺癌脑膜转移药物治疗的管理[J].中国肺癌杂志,2020,23(8):710-718.
[3]Nosaki K, Yamanaka T, Hamada A, et al. Erlotinib for Non-Small Cell Lung Cancer with Leptomeningeal Metastases: A Phase II Study (LOGIK1101). Oncologist. 2020 Dec;25(12):e1869-e1878. doi: 10.1634/theoncologist.2020-0640.
[4]Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget. 2015 Feb 28;6(6):4527-36. doi: 10.18632/oncotarget.2886.
[5]Arbour KC, Kris MG, Riely GJ, et al. Twice weekly pulse and daily continuous-dose erlotinib as initial treatment for patients with epidermal growth factor receptor-mutant lung cancers and brain metastases. Cancer. 2018 Jan 1;124(1):105-109. doi: 10.1002/cncr.30990.
[6]陆志琴,袁源亮,刘安文.非小细胞肺癌脑膜转移研究进展[J].肿瘤防治究,2020,47(12):986-991.
[7]Saboundji K, Auliac JB, Pérol M, et al. Efficacy of Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer with Leptomeningeal Metastases Pretreated with EGFR-Tyrosine Kinase Inhibitors. Target Oncol. 2018 Aug;13(4):501-507. doi: 10.1007/s11523-018-0581-2.
[8]Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for Patients With Leptomeningeal Metastases Associated With EGFR T790M-Positive Advanced NSCLC: The AURA Leptomeningeal Metastases Analysis. J Thorac Oncol. 2020 Apr;15(4):637-648. doi: 10.1016/j.jtho.2019.12.113.
[9]Yang JCH, Kim SW, Kim DW, et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol. 2020 Feb 20;38(6):538-547. doi: 10.1200/JCO.19.00457.
[10]Park S, Lee MH, Seong M, Kim ST, Kang JH, Cho BC, Lee KH, Cho EK, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol. 2020 Oct;31(10):1397-1404. doi: 10.1016/j.annonc.2020.06.017. Epub 2020 Jul 5. PMID: 32634610.
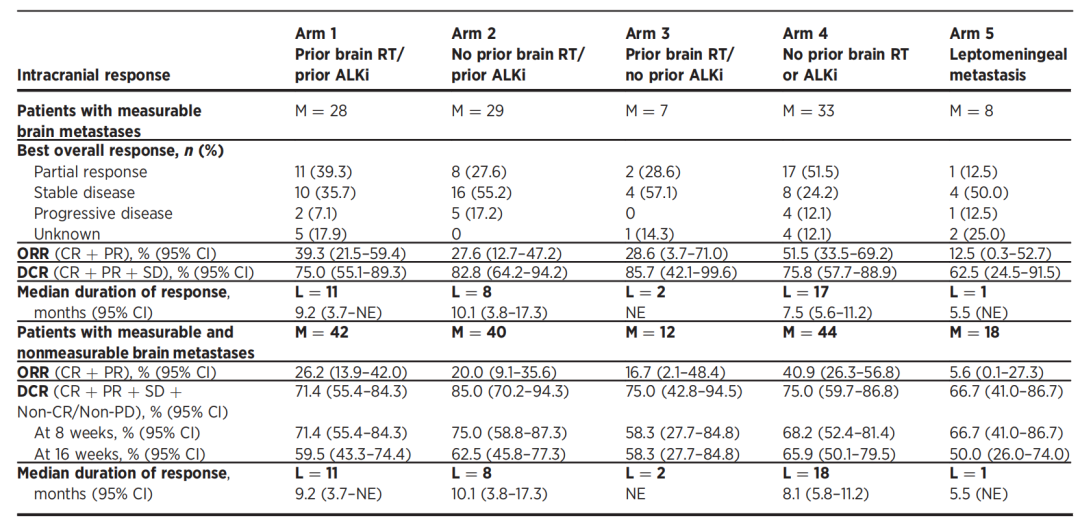
[11]Chow LQM, Barlesi F, Bertino EM, et al. ASCEND-7: Efficacy and Safety of Ceritinib Treatment in Patients with ALK-Positive Non-Small Cell Lung Cancer Metastatic to the Brain and/or Leptomeninges. Clin Cancer Res. 2022 Jun 13;28(12):2506-2516. doi: 10.1158/1078-0432.CCR-21-1838.
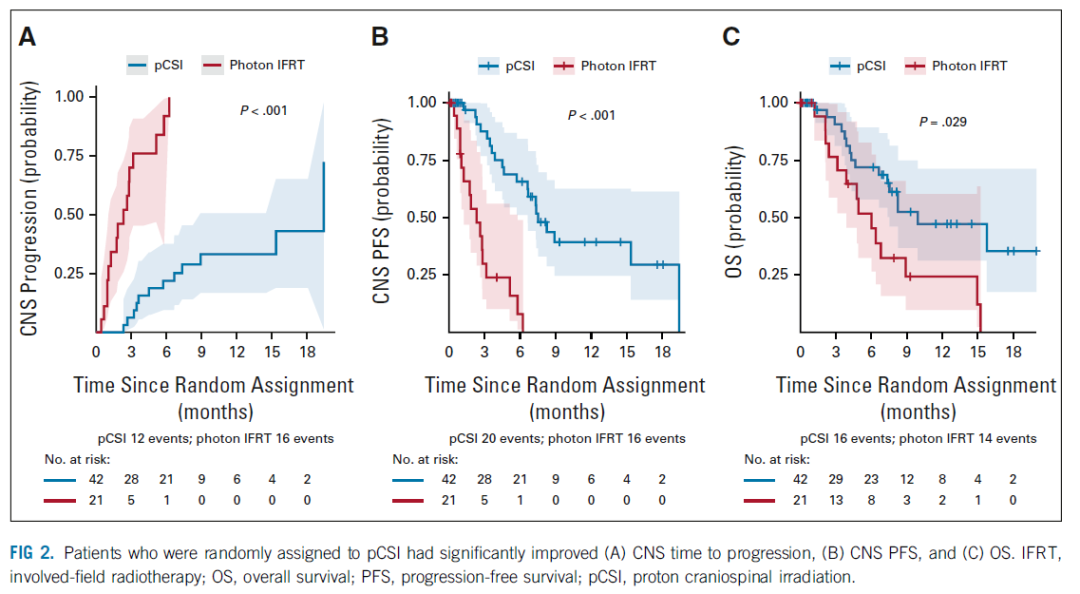
[12]Yang JT, Wijetunga NA, Pentsova E, et al. Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis. J Clin Oncol. 2022 Jul 8:JCO2201148. doi: 10.1200/JCO.22.01148.
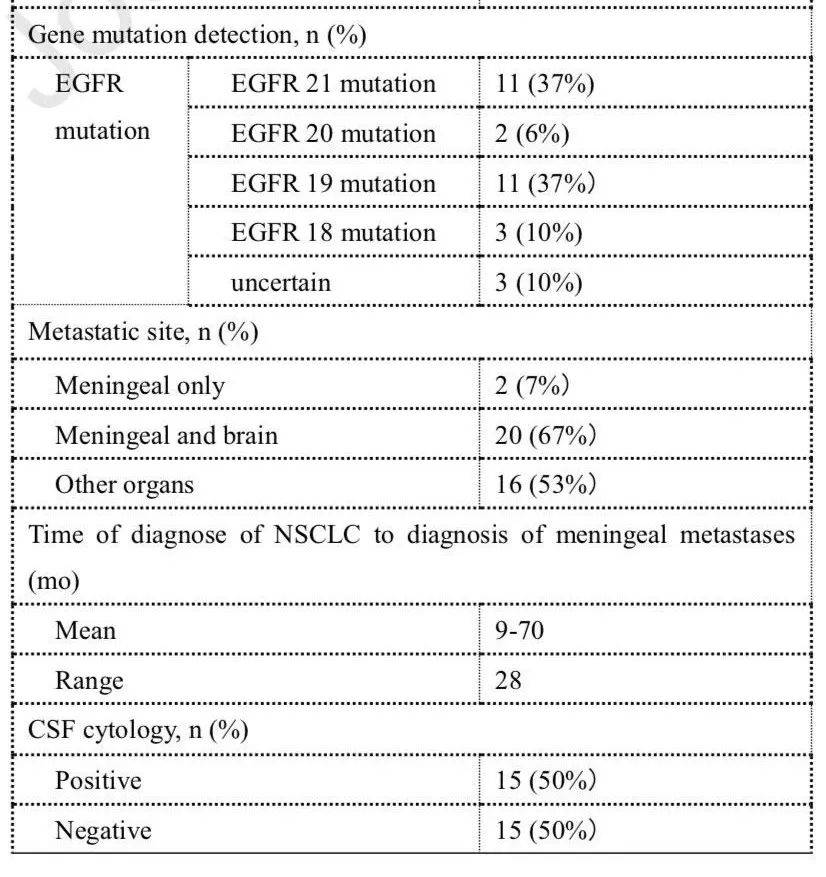
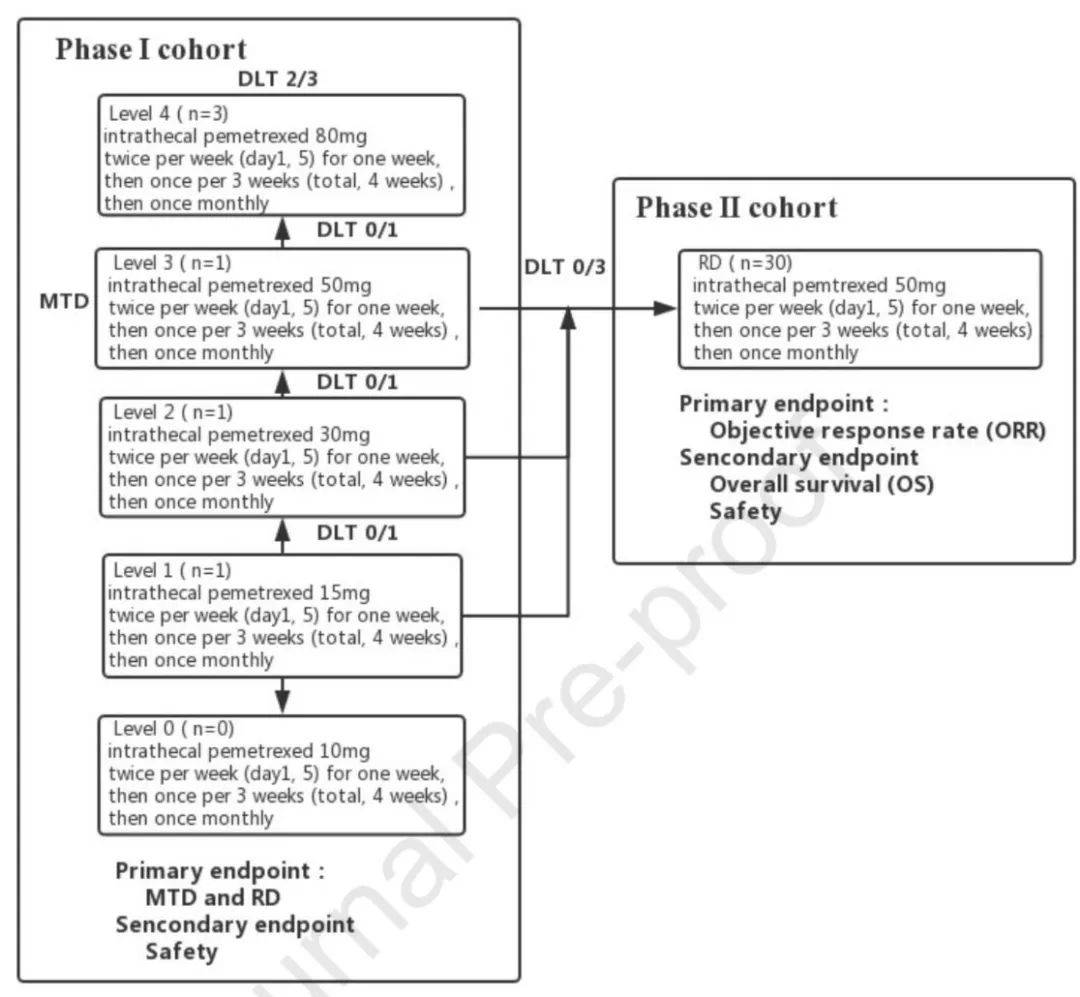
[13]Fan C, Zhao Q, Li L, et al. Efficacy and Safety of Intrathecal Pemetrexed Combined with Dexamethasone for Treating TKI-failed Leptomeningeal Metastases from EGFR-mutant NSCLC-A Prospective Open-label Single-arm Phase I/II Clinical Trial (unique identifier: ChiCTR1800016615). J Thorac Oncol. 2021 May 11:S1556-0864(21)02157-2. doi: 10.1016/j.jtho.2021.04.018.
[14]Choi M, Keam B, Ock CY,et al. Pemetrexed in the Treatment of Leptomeningeal Metastasis in Patients With EGFR-mutant Lung Cancer. Clin Lung Cancer. 2019 Jul;20(4):e442-e451. doi: 10.1016/j.cllc.2019.03.005.
[15]Hendriks LEL, Bootsma G, Mourlanette J, et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer. 2019 Jul;116:182-189. doi: 10.1016/j.ejca.2019.05.019.













 苏公网安备32059002004080号
苏公网安备32059002004080号


