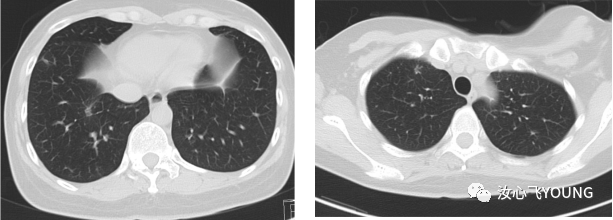
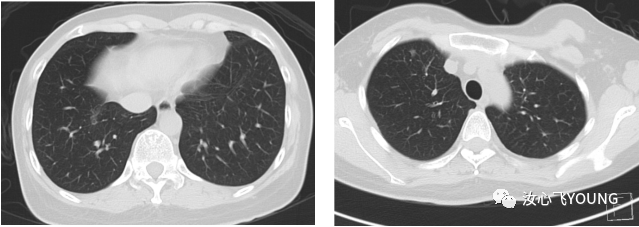
治疗概览

参考文献
[1] MIGLIETTA F, VISANI L, MARINI S, et al. Oligometastatic breast cancer: Dissecting the clinical and biological uniqueness of this emerging entity. Can we pursue curability? [J]. Cancer Treat Rev, 2022, 110(102462.
[2] SORAN A, OZMEN V, OZBAS S, et al. Randomized Trial Comparing Resection of Primary Tumor with No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01 [J]. Ann Surg Oncol, 2018, 25(11): 3141-9.
[3] BADWE R, HAWALDAR R, NAIR N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial [J]. The Lancet Oncology, 2015, 16(13): 1380-8.
[4] FITZAL F, BJELIC-RADISIC V, KNAUER M, et al. Impact of Breast Surgery in Primary Metastasized Breast Cancer: Outcomes of the Prospective Randomized Phase III ABCSG-28 POSYTIVE Trial [J]. Ann Surg, 2019, 269(6): 1163-9.
[5] JOHNSTON S R D, TOI M, O'SHAUGHNESSY J, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial [J]. The Lancet Oncology, 2023, 24(1): 77-90.
[6] SLAMON D J, FASCHING P A, HURVITZ S, et al. Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer [J]. Ther Adv Med Oncol, 2023, 15(17588359231178125.
[7] WANDER S A, HAN H S, ZANGARDI M L, et al. Clinical Outcomes With Abemaciclib After Prior CDK4/6 Inhibitor Progression in Breast Cancer: A Multicenter Experience [J]. J Natl Compr Canc Netw, 2021, 1-8.
[8] ANDRÉ F, CIRUELOS E, RUBOVSZKY G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer [J]. The New England journal of medicine, 2019, 380(20): 1929-40.
[9] RUGO H S, LEREBOURS F, CIRUELOS E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study [J]. The Lancet Oncology, 2021, 22(4): 489-98.
[10] ZHOU J, WU X, ZHANG H, et al. Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer [J]. Breast (Edinburgh, Scotland), 2022, 66(255-61.
[11] RUGO H S, BARDIA A, MARMÉ F, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer [J]. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2022, 40(29): 3365-76.
[12] MODI S, JACOT W, YAMASHITA T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer [J]. The New England journal of medicine, 2022, 387(1): 9-20.
病例提供

韩忠华 教授
福建医科大学附属协和医院
乳腺外科 副主任医师
医学博士,副教授,硕士研究生导师
海峡两岸医药卫生交流协会委员
海峡肿瘤防治科技交流协会委员
福建省抗癌协会肿瘤科普委员会委员
福建省抗癌协会肿瘤整形外科专业委员会委员
福建省抗癌协会肿瘤康复委员会青年委员会委员
福建省医学会科学普及分会委员青年委员
病例点评

林舜国 教授
福建医科大学附属协和医院
乳腺外科主任医师
中国抗癌协会乳腺癌专业委员会第五、六、七届委员
中国医师协会临床精准医疗专委会乳腺癌分会委员
海峽两岸医药卫生交流协会乳腺专业委员会执行主任委员
福建医学会外科分会乳腺专业组副组长
福建中医药学会乳腺病分会副主任委员
福建医学会科学普及分会副主任委员













 苏公网安备32059002004080号
苏公网安备32059002004080号


